(2: 内蒙古农业大学动物科学学院, 呼和浩特 010018)
(3: 中国科学院南京地理与湖泊研究所湖泊与环境国家重点实验室, 南京 210008)
(4: 中国科学院大学, 北京 100049)
(2: College of Animal Science, Inner Mongolia Agricultural University, Huhehaote 010018, P. R. China)
(3: State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, P. R. China)
(4: University of Chinese Academy of Sciences, Beijing 100049, P. R. China)
寡毛类(如霍甫水丝蚓(Limnodrilus hoffmeisteri))是富营养水体底栖动物典型优势种, 该种类生物能够通过摄食、匍行、筑穴、钻孔等形式对沉积物颗粒产生搬运和混合,从而促进沉积物中营养盐向上覆水释放,这些营养盐被浮游植物利用后会使得水体维持一个较高的浊度,从而不利于湖泊生态系统的藻-草型转换[1].河蚬(Corbicula fluminea)属软体动物双壳纲,穴居于淡水及咸淡水底沉积物表层[2],广泛分布在我国的淡水湖泊、河流、水库等水体.研究发现河蚬滤食能显著降低浮游植物密度,增加水体透明度,进而有利于沉水植物恢复[3].通过放养河蚬或蚌等土著双壳类滤食动物来改善水质也是富营养湖泊生态修复的常用手段之一.到目前为止,对于寡毛类和双壳类对沉积物-水界面的营养盐循环都已经进行了大量的研究,结果显示寡毛类(如水丝蚓)生物扰动对于沉积物内源释放具有促进作用[4-6],而河蚬则具有降低水体叶绿素a浓度、改善水质的功能[7-9],但是对于二者之间是否存在交互作用还有待阐明.河蚬与霍甫水丝蚓均为太湖底栖动物优势种[10],在2个种群的交错区,二者共同作用会使营养盐循环产生何种变化?河蚬能否抑制霍甫水丝蚓生物扰动导致的水体营养盐浓度升高?为此,本文设计了两因素的受控实验,以河蚬和霍甫水丝蚓为研究对象,探讨河蚬在“水丝蚓—营养盐释放”过程中的作用,以期为富营养湖泊的生态修复提供参考依据.
1 材料与方法实验容器为封底有机玻璃柱(内径14 cm,深度50 cm).每柱加入深度为15 cm经200 μm筛网过滤且充分混匀的湖泊表层沉积物,随后沿壁缓缓注入经450目筛网过滤后的湖水,尽量避免注水期间对表层沉积物的扰动,实验所用沉积物、湖水均取自太湖梅梁湾.
实验设2个影响因子共4种处理,分别为对照组、河蚬组、霍甫水丝蚓组和混合组(霍甫水丝蚓和河蚬),每种处理设4个重复,共有机玻璃柱16个.为了减缓实验水温波动,有机玻璃柱露天放置于4个注满水的蓝色方缸(长×宽×高为68 cm×53 cm×38.5 cm)内. 2014年9月15日按上述方法往有机玻璃柱中加入沉积物和水样,静置一周后挑选体长和活性相似的霍甫水丝蚓8份(150条/组),分别加到混合组和水丝蚓组中,同时称取壳长和生物量相近的河蚬8份(4只/组),分别加到混合组和河蚬组中,稳定一夜后于2014年9月22日正式开始实验.实验前一周将采自采泥点的霍甫水丝蚓和河蚬分别暂养于2 L的烧杯和白色方缸(长×宽×高为122 cm×71 cm×62 cm)中备用.河蚬和霍甫水丝蚓的密度设置参照2007-2008年太湖的野外调查结果[10].
实验期间每次采样后补加同体积纯净水,为抑制管壁附着生物的生长,每2天利用软毛刷轻轻刷除管壁附着生物.实验期间平均水温为18.7℃,范围16.9~20.6℃.
实验持续20 d,分别于0、5、10、15、20 d采样测定水体总氮(TN)、总磷(TP)和叶绿素a(Chl.a)浓度,方法依据《湖泊富营养化调查规范》[11];最后一次采样用内径为1.6 cm的有机玻璃管采集厚度约为1 cm的表层沉积物,测定底栖藻叶绿素a浓度,方法参照水体叶绿素a浓度测定方法热乙醇法;同时测定水体悬浮物(TSS)浓度,方法参照《湖泊富营养化调查规范》[11]. TN、TP和Chl.a采用重复测量方差分析(repeated-measures two-way ANOVA)进行比较,水体悬浮物(TSS)及底栖藻叶绿素a浓度采用两因素方差分析(two-way ANOVA)进行比较,数据统计分析均采用SPSS 19.0软件.
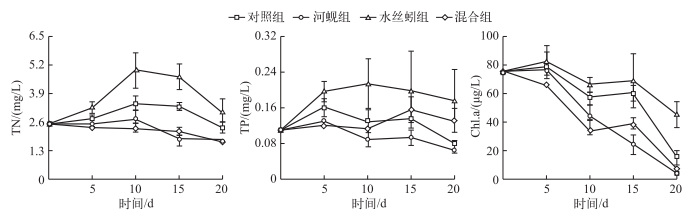
2 结果 2.1 水体总氮、总磷和叶绿素a浓度霍甫水丝蚓、河蚬均对水体TN浓度具有显著影响(P < 0.01),且二者间存在交互作用(P < 0.01).实验结束时,河蚬组TN浓度比对照组降低了24.3%;添加河蚬后,混合组TN浓度比霍甫水丝蚓组降低了43.0%.霍甫水丝蚓能显著增加水体TN浓度,实验结束时,霍甫水丝蚓组TN浓度与对照组相比增加了27.8%.
河蚬和霍甫水丝蚓均对水体TP浓度具有显著影响(P < 0.05),二者间无交互作用(P > 0.05).河蚬能显著降低水体TP浓度,实验结束时,河蚬组TP浓度与对照组相比降低了14.1%.霍甫水丝蚓能显著增加水体TP浓度,实验结束时,霍甫水丝蚓组TP浓度为对照组的1.2倍.
|
图 1 水体中TN、TP和Chl.a浓度的时间变化(平均值±标准误) Fig.1 Temporal changes of TN, TP and Chl.a concentrations in water column |
| 表 1 水体TN、TP、Chl.a重复测量方差分析及TSS和底栖藻叶绿素a两因素方差分析结果 Tab.1 Analysis of repeated-measures two-way ANOVA results for TN, TP, Chl.a and analysis of two-way ANOVA results for TSS and Chl.a concentration of benthic algae |
河蚬与霍甫水丝蚓均对水体Chl.a浓度具有显著影响(P < 0.05),二者间存在交互作用(P < 0.05).实验结束时,河蚬组Chl.a浓度与对照组相比降低了73.3%,添加河蚬后,混合组Chl.a浓度比霍甫水丝蚓组降低了83.3%.霍甫水丝蚓能显著增加水体Chl.a浓度,实验结束时,霍甫水丝蚓组Chl.a浓度是对照组的1.92倍.
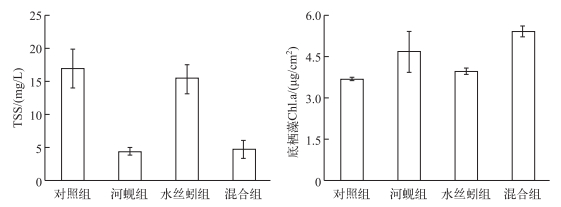
2.2 水体悬浮物和底栖藻叶绿素a浓度河蚬对TSS及底栖藻叶绿素a浓度具有显著影响(P < 0.01),实验结束时,河蚬组中TSS浓度与对照组相比降低了73.8%,而底栖藻叶绿素a浓度显著高于对照组26.8%.霍甫水丝蚓对TSS及底栖藻叶绿素a浓度无显著影响(P > 0.05),二者间无交互作用(P > 0.05)(图 2).
|
图 2 实验结束时水体中悬浮物和底栖藻叶绿素a浓度(平均值±标准误) Fig.2 The TSS concentration in water column and the Chl.a concentration of benthic algae measured at the end of experiment |
研究结果表明霍甫水丝蚓能显著增加水体总氮、总磷浓度,这与Zhang等[12]、吴方同等[6, 13]的研究结果一致.水丝蚓的栖居方式是将身体的前端埋于底泥中,后端露于水中且不停摆动进行呼吸,产生的垂向通道有助于上覆水和间隙水相互交换,促进沉积物中的氮、磷向水中释放[4, 14].水丝蚓的摄食和排泄将有机物转化为无机物,也促进了氮、磷向水体中释放[15-16].此外,底栖动物的生物扰动还会显著提高沉积物中微生物的矿化活动,提高营养物的再生效率[17-18],而沉积物中溶出的氮磷最终通过生物扩散[20]释放到水体中,进而增加水体总氮、总磷浓度.在本研究中实验前10 d水体总氮、总磷浓度均呈上升趋势,之后开始出现下降,一方面可能是因为浮游藻类发生沉降,另一方面则可能是因为管壁附着生物固定了部分氮、磷.
霍甫水丝蚓的大量存在可能会进一步加剧湖泊的富营养化程度.水丝蚓能耐受由于有机物大量被分解而造成的低氧甚至缺氧环境,故而能在富营养严重的水体生存,而其它底栖动物在这种环境下往往受到抑制甚至死亡[21],这就使得其在富营养化水体中易成为优势底栖种.本研究显示, 水丝蚓能显著促进内源释放,且对浮游藻类的生长具有显著促进作用,由此推测水丝蚓对富营养化存在一定的正反馈效应.
本研究结果显示, 河蚬能显著降低水体总氮、总磷及叶绿素a浓度.类似地,Hwang等[8]研究发现,在高密度河蚬的处理中,上覆水叶绿素浓度持续从87 mg/L降至25 mg/L,同时水体悬浮物及总磷浓度也显著降低. Welker等[22]也发现双壳类能显著降低浮游植物生物量和总磷浓度,此外徐海军等[9]发现河蚬对藻类的消除率可达88%±3.1%.水体氮、磷浓度显著降低的主要原因,一方面是河蚬对浮游植物和颗粒物的高强度滤食[23], 另一方面,河蚬对底栖藻类的生长具有促进作用,抑制了沉积物营养释放,从而间接降低水体总氮、总磷浓度.研究表明底栖藻类生物量与光照强度存在正相关[24],河蚬的滤食和生物沉降作用能增加水体透明度,从而增加底栖藻类的生物量,进而使底栖藻类对营养盐的直接吸收和固定作用增强[25-26],最终减少水体氮磷浓度.而河蚬对于水体叶绿素a浓度的降低作用主要是由其滤食作用所致.研究表明食物质量和颗粒物粒径大小会对滤食产生影响[27].本研究中浮游植物主要由蓝藻门的弯形小尖头藻(Raphidiopsis curvata)、束丝藻(Aphanizomenon sp.)和裸藻门的裸藻(Euglena sp.)构成,分别占整个浮游植物群落的87.5%、12.4%和0.06%(未发表数据),一方面,蓝藻作为一种低质量食物[28],河蚬作为非选择性滤食者[29]其为获得足够的营养可能会加强对蓝藻的滤食; 另一方面,3种藻类的粒径不会对河蚬的鳃造成堵塞而影响其滤食过程,从而使水体叶绿素a浓度显著下降.此外,底栖藻类在抑制沉积物营养释放的同时,也能从水体中吸收营养,从而抑制了浮游藻类生长,致使水体叶绿素a浓度下降.
河蚬能够显著减弱水丝蚓对总氮和叶绿素a浓度的增加作用,表明在水丝蚓分布区投加河蚬应能起到改善富营养水体的作用. Howard等[30]研究发现贝类的生物沉降物富含营养物质且易于同化,故河蚬的排泄及滤食可能为水丝蚓提供额外食物来源,从而减弱其为获取食物而进行的掘穴活动所带来的对沉积物的扰动,Lewandowski等[31]研究发现水丝蚓可钻到沉积物—水界面下20 cm,故而掘穴活动的减弱,会减少上覆水与深层沉积物的进一步接触,从而减弱深层沉积物中营养物质的释放,从而使水体总氮浓度显著降低.而叶绿素a浓度的降低主要是河蚬对浮游藻类的滤食作用超过水丝蚓营养释放造成的浮游藻类生长所致.本研究中水丝蚓密度约为9700 ind./m2,而河蚬密度约为260 ind./m2(太湖平均密度),在此密度设置下,河蚬能对富营养化水质起到改善作用,但有研究表明在富营养化严重的局部区域水丝蚓密度可大于60000 ind./m2[32],在水丝蚓极高密度区域由于底泥有机质含量极高,河蚬能否存活并具有水质改善作用则有待进一步研究.
4 结论1) 水丝蚓生物扰动能显著增加水体总氮和总磷浓度,对浮游藻类生长具有促进作用.
2) 河蚬能通过滤食和生物沉降作用降低水体总氮、总磷、叶绿素a及悬浮物浓度,提高水体透明度,进而促进底栖藻类的生长,同时对水丝蚓生物扰动所致的沉积物内源释放具有抑制作用.
3) 河蚬和水丝蚓对水体总氮和叶绿素a浓度存在交互作用,河蚬削弱了水丝蚓对总氮和叶绿素a浓度的增加作用.
| [1] |
Reise K. Tidal flat ecology. An experimental approach to species interactions. Berlin Heidelberg: Springer-Verlag, 1985.
|
| [2] |
Zhang Hucai, Chen Yue, Fan Hongfang et al. Climatic background of modern Corbicula fluminea and the stable isotopes of shells from the reprensentative areas in continental China. Marine Geology & Quaternary Geology, 2007, 27(3): 77-84. [张虎才, 陈玥, 樊红芳等. 河蚬分布的气候环境及壳体稳定同位素. 海洋地质与第四纪地质, 2007, 27(3): 77-84.] |
| [3] |
Caraco NF, Cole JJ, Strayer DL. Top down control from the bottom:Regulation of eutrophication in a large river by benthic grazing. Limnology and Oceanography, 2006, 51(1part2): 664-670. DOI:10.4319/lo.2006.51.1_part_2.0664 |
| [4] |
Bai Xiuling, Zhou Yunkai, Zhang Lei. The influence of tubificid worms bioturbation on organic phosphorus components and their vertical distribution in sediment of Lake Taihu. Acta Ecologica Sinica, 2012, 32(17): 5581-5588. [白秀玲, 周云凯, 张雷. 水丝蚓对太湖沉积物有机磷组成及垂向分布的影响. 生态学报, 2012, 32(17): 5581-5588. DOI:10.5846/stxb201110231569] |
| [5] |
Zhang Lei, Gu Xiaozhi, Wang Zhaode et al. The influence of Tubificid worms bioturbation on the exchange of phosphorus across sediment-water interface in lakes. J Lake Sci, 2010, 22(5): 666-674. [张雷, 古小治, 王兆德等. 水丝蚓(Tubificid worms)扰动对磷在湖泊沉积物-水界面迁移的影响. 湖泊科学, 2010, 22(5): 666-674. DOI:10.18307/2010.0507] |
| [6] |
Wu Fangtong, Chen Jinxiu, Yan Yanhong et al. The influence of Limnodrilus hoffmeisteri bioturbation on nitrogen release from sediments in the East Lake Dongting. J Lake Sci, 2011, 23(5): 731-737. [吴方同, 陈锦秀, 闫艳红等. 水丝蚓生物扰动对东洞庭湖沉积物氮释放的影响. 湖泊科学, 2011, 23(5): 731-737. DOI:10.18307/2011.0510] |
| [7] |
Zhu Xiaolong, Gu Jiao, Jin Hui et al. Effects of Corbicula fluminea in Lake Taihu on improvement of eutrophic water quality. J Lake Sci, 2015, 27(3): 486-492. [朱小龙, 谷娇, 靳辉等. 太湖河蚬(Corbicula fluminea)对富营养水体水质的改善作用. 湖泊科学, 2015, 27(3): 486-492. DOI:10.18307/2015.0316] |
| [8] |
Hwang SJ, Kim HS, Park JH et al. Shift in nutrient and plankton community in eutrophic lake following introduction of a freshwater bivalve. Journal of Environmental Biology, 2011, 32(2): 227-234. |
| [9] |
Xu Haijun, Lin Qufei, Yang Caigen et al. Preliminary studies on the elimination effect of algae by three species of freshwater bivalve. Journal of Hydroecology, 2010, 3(1): 72-73. [徐海军, 凌去非, 杨彩根等. 3种淡水贝类对藻类消除作用的初步研究. 水生态学杂志, 2010, 3(1): 72-73.] |
| [10] |
Cai Yongjiu, Gong Zhijun, Qin Boqiang. Community structure and diversity of macrozoobenthos in Lake Taihu, a large shallow eutrophic lake in China. Biodiversity Science, 2010, 18(1): 50-59. [蔡永久, 龚志军, 秦伯强. 太湖大型底栖动物群落结构及多样性. 生物多样性, 2010, 18(1): 50-59.] |
| [11] |
Jin Xiangcan, Tu Qingying eds. Lake eutrophication investigation specification:The second edition. Beijing: China Environmental Science Press, 1990. [金相灿, 屠清瑛. 湖泊富营养化调查规范(第二版). 北京: 中国环境科学出版社, 1990.]
|
| [12] |
Zhang X, Liu Z, Jeppesen E et al. Effects of deposit-feeding tubificid worms and filter-feeding bivalves on benthic-pelagic coupling:Implications for the restoration of eutrophic shallow lakes. Water Research, 2014, 50(3): 135-146. |
| [13] |
Wu Fangtong, Yan Yanhong, Sun Shiquan et al. Influence of Limnodrilus hoffmeisteri bioturbation on phosphorus release from sediment. Chinese Journal of Environmental Engineering, 2011, 5(5): 1071-1076. [吴方同, 闫艳红, 孙士权等. 水丝蚓生物扰动对沉积物磷释放的影响. 环境工程学报, 2011, 5(5): 1071-1076.] |
| [14] |
Hedman JE, Gunnarsson JS, Samuelsson G et al. Particle reworking and solute transport by the sediment-living polychaetes Marenzelleria neglecta and Hediste diversicolor. Journal of Experimental Marine Biology and Ecology, 2011, 407(2): 294-301. DOI:10.1016/j.jembe.2011.06.026 |
| [15] |
Devine JA, Vanni MJ. Spatial and seasonal variation in nutrient excretion by benthic invertebrates in a eutrophic reservoir. Freshwater Biology, 2002, 47(6): 1107-1121. DOI:10.1046/j.1365-2427.2002.00843.x |
| [16] |
Ji L, Song C, Cao X et al. Spatial variation in nutrient excretion by macrozoobenthos in a Chinese large shallow lake(Lake Taihu). Journal of Freshwater Ecology, 2015, 30(1): 169-180. DOI:10.1080/02705060.2014.997816 |
| [17] |
Banta GT, Andersen O. Bioturbation and the fate of sediment pollutants:experimental case studies of selected infauna species. Vie et Milieu, 2003, 53(4): 233-248. |
| [18] |
Guo Liang, Yao Sipeng, Xin Peng. The influence of Limnodrilus hoffmeisteri bioturbation on the bacterial community composition and diversity in surface sediment. Journal of Agro-Environment Science, 2011, 30(5): 973-978. [郭亮, 姚思鹏, 邢鹏. 霍甫水丝蚓(Limnodrilus hoffmeisteri)扰动对表层沉积物细菌群落结构和多样性的影响. 农业环境科学学报, 2011, 30(5): 973-978.] |
| [19] |
Wang Xue, Zhao Dayong, Zeng Jin et al. Effects of Corbicula fluminea bioturbation on the community composition and abundance of ammonia-oxidizing archaea and bacteria in Surface Sediments. Environmental Science, 2014, 35(6): 2314-2321. [王雪, 赵大勇, 曾巾等. 河蚬(Corbicula fluminea)扰动对表层沉积物中氨氧化菌群落结构和丰度的影响. 环境科学, 2014, 35(6): 2314-2321. DOI:10.13227/j.hjkx.2014.06.038] |
| [20] |
Koretsky CM, Meile C, Van Cappellen P. Quantifying bioirrigation using ecological parameters:a stochastic approach. Geochem Trans, 2002, 3(3): 17-30. DOI:10.1039/b110459d |
| [21] |
Gong Zhijun, Xie Ping, Tang Huijuan et al. The influence of eutrophycation upon community structure and biodiversity of macrozoobenthos. Acta Hydrobiologica Sinica, 2001, 25(3): 210-216. [龚志军, 谢平, 唐汇涓等. 水体富营养化对大型底栖动物群落结构及多样性的影响. 水生生物学报, 2001, 25(3): 210-216.] |
| [22] |
Welker M, Walz N. Can mussels control the plankton in rivers?-a planktological approach applying a Lagrangian sampling strategy. Limnology and Oceanography, 1998, 43(5): 753-762. DOI:10.4319/lo.1998.43.5.0753 |
| [23] |
Strayer DL, Caraco NF, Cole JJ et al. Transformation of freshwater ecosystems by bivalves. BioScience, 1999, 49(1): 19-27. DOI:10.2307/1313490 |
| [24] |
Yao Yang, Jin Xiangcan, Jiang Xia et al. Study on effects of light on phosphorus release and phosphorus form change in lake sediments. Research of Environmental Sciences, 2004, 17(z1): 30-33. [姚扬, 金相灿, 姜霞等. 光照对湖泊沉积物磷释放及磷形态变化的影响研究. 环境科学研究, 2004, 17(z1): 30-33. DOI:10.3321/j.issn:1001-6929.2004.z1.007] |
| [25] |
Wetzel RG. Limnology:lake and river ecosystems. Gulf Professional Publishing, 2001.
|
| [26] |
Zhang X, Liu Z, Gulati RD et al. The effect of benthic algae on phosphorus exchange between sediment and overlying water in shallow lakes:A microcosm study using 32P as a tracer. Hydrobiologia, 2013, 710(1): 109-116. DOI:10.1007/s10750-012-1134-9 |
| [27] |
Vanderploeg HA, Liebig JR, Carmichael WW et al. Zebra mussel(Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay(Lake Huron) and Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences, 2001, 58(6): 1208-1221. DOI:10.1139/f01-066 |
| [28] |
Basen T, Martin-Creuzburg D, Rothhaupt KO. Role of essential lipids in determining food quality for the invasive freshwater clam Corbicula fluminea. Journal of the North American Benthological Society, 2011, 30(3): 653-664. DOI:10.1899/10-087.1 |
| [29] |
Boltovskoy D, Izaguirre I, Correa N. Feeding selectivity of Corbicula fluminea (Bivalvia) on natural phytoplankton. Hydrobiologia, 1995, 312(3): 171-182. DOI:10.1007/BF00015510 |
| [30] |
Howard JK, Cuffey KM. The functional role of native freshwater mussels in the fluvial benthic environment. Freshwater Biology, 2006, 51(3): 460-474. DOI:10.1111/j.1365-2427.2005.01507.x |
| [31] |
Lewandowski J, Hupfer M. Effect of macrozoobenthos on two-dimensional small-scale heterogeneity of pore water phosphorus concentrations in lake sediments:A laboratory study. Limnology and Oceanography, 2005, 50(4): 1106-1118. DOI:10.4319/lo.2005.50.4.1106 |
| [32] |
Wavre M, Brinkhurst RO. Interactions between some tubificid oligochaetes and bacteria found in the sediments of Toronto Harbour, Ontario. Journal of the Fisheries Board of Canada, 1971, 28(3): 335-341. DOI:10.1139/f71-045 |
 2016, Vol. 28
2016, Vol. 28 


