(2: 山东省环境科学工程技术研究中心, 济南 250061)
(2: Shandong Provincial Engineering Center on Environmental Science and Technology, Jinan 250061, P.R.China)
近年来,由于水体富营养化,蓝藻水华在湖泊、水库及河流等水体中频繁暴发,不仅对当地水生生态系统平衡造成一定破坏,而且某些产毒蓝藻的大规模扩散,还会导致水体中毒素浓度升高,当被污染水体作为饮用水水源或娱乐水源时,会对人类和动物的健康造成严重危害[1-2].在世界范围内,可形成水华的蓝藻除最常见的微囊藻、鱼腥藻和束丝藻之外[2],拟柱孢藻(Cylindrospermopsis raciborskii)作为能产生毒素的另一蓝藻种类,由于其毒性、暴发性和入侵性而日益受到关注[3-5].拟柱孢藻对氮、磷营养盐的利用效率高,对温度和光照的适应性强,在高温、富营养化的湖泊和水库中可快速生长,频繁形成水华[6-7].
拟柱孢藻可产生柱孢藻毒素(cylindrospermopsin,CYN)和麻痹性贝类毒素(paralytic shellfish poisoning,PSP)等有毒物质[8-10],其中CYN作为一种肝毒素,能导致肝脏和肾脏受损,并可对DNA和RNA产生损伤[9, 11-13].随着全球气候变暖以及水体富营养化程度的加剧,拟柱孢藻呈现出显著的全球扩张趋势,其种群分布不断由热带、亚热带向温带地区扩张,全球诸多地区的淡水饮用水水源地相继检测到拟柱孢藻的存在,且具有成为优势种的潜能.
拟柱孢藻在全球范围内的快速扩张,无疑将对众多淡水水源地造成严重威胁,因此有必要对该藻进行深入研究以更好地了解其生理特征,从而有效控制和消除该藻对人类的潜在危害.目前,人们对其地域扩张、生长条件和产毒特性等进行了初步探索研究,但国内学者对其关注和研究较少.此外,有效检测和去除拟柱孢藻及其毒素对保证饮用水安全具有十分重要的意义,也相继受到了各国环境研究者的关注.
本文主要从拟柱孢藻的分布、生态生理特征、产毒种类及毒性、检测和去除等几个方面进行概述,并对相关领域进行展望,为拟柱孢藻的进一步研究提供参考.
1 全球分布在全球范围内,拟柱孢藻目前主要分布在热带、亚热带地区,并逐渐向温带地区扩散[14-15].早在1899-1900年,人们在印度尼西亚爪哇岛首次发现拟柱孢藻[16],1939年在印度和其他热带地区也发现了它的存在[17],因而人们初步判定其为一株热带藻种(表 1).此后,诸多报道相继证明拟柱孢藻在热带、亚热带和温带地区以及除南极洲以外的所有大洲均有分布[3],其全球分布情况如图 1所示.
| 表 1 拟柱孢藻出现地区汇总 Tab.1 A region summary of Cylindrospermopsis raciborskii occurrence |

|
图 1 全球拟柱孢藻分布(修改自参考文献[3]) Fig.1 Global distribution of Cylindrospermopsis raciborskii (●拟柱孢藻,*拟柱孢藻可产CYN及CYN类似物,♦拟柱孢藻可产PSP) |
在国内,拟柱孢藻广泛分布于广东省的多处水库和湖泊中[18-23],并在鹤地、高州等水库及多个虾池中检测到其水华的暴发[7, 24].此外,福建[25]、云南[26-27]、湖北[28-29]、台湾[30]、太湖流域[31]以及山东[29]等地也均检测到拟柱孢藻的存在.
1979年澳大利亚昆士兰发生的“帕门岛神秘疾病”事件使人们认识到拟柱孢藻存在产毒藻种[9].由于接触了拟柱孢藻污染的水体,当地148名居民表现出中毒现象,并伴有肠胃炎的症状[8],随后研究人员发现从这些水体中分离出来的拟柱孢藻具有高毒性,并将其产生的化合物命名为柱孢藻毒素[32].截至目前,产毒拟柱孢藻主要分布于澳大利亚[5, 9]、新西兰[33]、亚洲的东部和东南部地区[23-34]以及沙特阿拉伯地区[35].
目前,研究人员针对拟柱孢藻的分布和扩张提出的假说主要有两种[36]:其一是非洲中心说,即认为拟柱孢藻起源于非洲的热带湖泊,随后扩散到赤道附近的印度尼西亚和中美洲等地区;其二是澳大利亚中心说,即由澳大利亚散布到其他热带、亚热带及温带地区.以上推断都是在流行病学、水文数据和物种生理特征的分析基础之上建立起来的.目前,众多学者正通过对不同区域的藻种进行基因数据分析来推断其相似性以进一步验证假说的科学性[4, 14, 37-38].此外,人们对于拟柱孢藻扩张到温带地区的具体原因,尚未达成共识.根据目前文献介绍[3, 5, 14],推断拟柱孢藻不断向温带地区扩张的原因主要为:水体富营养化程度的加剧及全球气候变暖;拟柱孢藻对温度及氮磷等外部条件的适应性强;不同区域之间的船舱运输、鸟类传播等.
2 生态生理特征 2.1 形态特征拟柱孢藻为蓝藻类原核生物,属于念珠藻目念珠藻科拟柱孢藻属,以拉式拟柱孢藻(Cylindrospermopsis raciborskii)为模式种[86].
拟柱孢藻是一种丝状藻,整条藻丝粗细均匀,但不同地区形态大小各具差异.本实验室拍摄到拟柱孢藻(分离自山东省某水库)形态如图 2所示.藻丝通常宽2~5 μm,长度变化范围大(图 2),常介于10~1000 μm之间[87-88],大多数为直线形,某些地区的藻丝呈卷曲形[71, 89];拟柱孢藻的单个细胞长度约为3~10 μm,由于其很少出现细胞收缩,单个细胞往往较难区分[88];在其生长旺盛期,可观测到伪空胞,为其在水体中提供浮力,以获得适宜的生长条件;藻丝末端有时可见异形胞(图 2A、D),数目少而不定,具有固氮功能.野生拟柱孢藻常有异形胞,而实验室人工培养的拟柱孢藻少见异形胞且藻体较大. Plominsky等[90]实验研究发现,在缺乏无机氮源的条件下,拟柱孢藻可产生异形胞进行固氮作用,当继续供给无机氮源时,约5 d后异形胞又会自行消失.此外,当出现环境胁迫时,拟柱孢藻可形成厚壁孢子(图 2),且孢子一般位于藻丝中部和尾部之间,每个个体的孢子数量一般为1~4个[89].
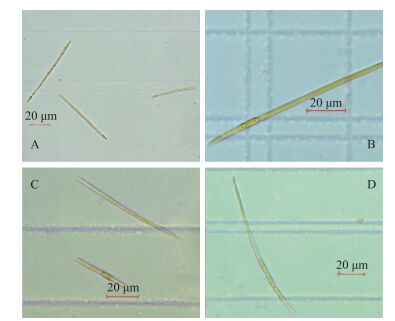
|
图 2 拟柱孢藻常见形态(A:具有末端异形胞; B:具有一个靠近末端的厚壁孢子(无异形胞); C:一条具有一个靠近藻丝中部的厚壁孢子(无异形胞),另一条既无厚壁孢子也无异形胞; D:具有一个末端异形胞和三个靠近藻丝末端的厚壁孢子(不靠近异形胞)) Fig.2 Common morphology of Cylindrospermopsis raciborskii |
随着全球气候变暖程度的加深,拟柱孢藻的生长范围逐渐扩大[5, 36].拟柱孢藻可在热带和亚热带水体中成为优势藻种,在夏季容易形成水华,当温带地区气温较高时(夏季)可在水体中占优势[23, 91-92].拟柱孢藻适合在温度为20~35℃条件下生长,最适生长温度约30℃[93],适宜高温生长的特点表明它是最可能从全球气候变暖中受益的蓝藻之一.拟柱孢藻水华既可在分层的、较深的水库(>15 m)中暴发[92],也可在浅水中暴发[81, 91].由于拟柱孢藻含有伪空胞,在水体中不易下沉,可在水体中自由浮动,所以其生长对光照要求不高,多项研究表明,拟柱孢藻的暴发与水体是否分层无关[94-95].
2.3 生长影响因素 2.3.1 营养盐的影响拟柱孢藻呈现多样化的营养策略,对氮、磷营养盐的利用效率高.拟柱孢藻能够适应不同的氮源,在低氮条件下可产生异形胞来固定空气中的氮气,且能有效利用有机氮[96-97].实验研究发现,当供给氮源为NH4+时藻丝生长速率最快,其次为NO3-,最后为尿素氮[98-99].在产毒方面,不同藻株产毒情况受氮浓度影响不同.例如,对于产生CYN的拟柱孢藻藻株(Woloszynska),有研究表明,当氮源匮乏时会促进CYN的生成,相反地,当提供充足氮源(尤其是NH4+)时,CYN浓度降低[100];而对于产生蛤蚌毒素的拟柱孢藻藻株(分离自巴西),Brentano等[101]发现随着溶解性无机氮的增加,其产生的蛤蚌毒素浓度增大.为此,拟柱孢藻的具体产毒机理与氮源之间的关系仍需进一步探究.
拟柱孢藻对磷有很强的吸收和储存能力,在磷营养不足的环境下容易成为优势藻种,当湖泊或水库中磷浓度较低时,拟柱孢藻更易形成水华[97].拟柱孢藻相比微囊藻、束丝藻具有更高的胞外磷酸酶活性,在缺乏无机磷源的环境下可以充分利用有机磷源[94, 97].最近,Willis等[102]在增加磷浓度的条件下发现拟柱孢藻生长速率下降,猜测磷会被优先储存而非用于其生长.另有研究发现,在磷限制的情况下,CYN产生率与其指数生长期的生长率呈正相关[103].
此外,戴景峻等[104]发现氮对拟柱孢藻N8的生长限制作用比磷更为显著,低氮显著限制拟柱孢藻N8的生长,这种抑制不因磷浓度的升高而解除,相反,氮浓度的升高可延长拟柱孢藻在磷限制性条件下的生长时间.而有研究表明,低的氮磷比能诱导湖泊中固氮蓝藻的形成[105],因此,不同氮磷比对拟柱孢藻的影响情况有待进一步研究.
近年来,关于氮、磷营养元素对拟柱孢藻影响的研究逐渐增多,这对了解拟柱孢藻的营养利用策略和预测拟柱孢藻在不同营养条件下暴发的可能性具有重要意义.目前,关于氮、磷对其生长的影响机理研究尚不充分.尤其关于N、P等营养元素对拟柱孢藻产毒的影响研究较少,例如,关于氮磷对其产毒的具体影响机制尚不明确,仍需要对不同区域藻种做一步研究.
2.3.2 温度及光照的影响拟柱孢藻对温度和光照的适应性较强,能够适应环境中波动的温度和光照条件,但温度和光照等环境因子对拟柱孢藻的形态变化和产毒也有一定影响[64, 87, 89, 106].研究表明,即使在温度低至16℃时,拟柱孢藻仍可保持一定的生物量[107],Bonilla等[108]发现拟柱孢藻甚至可以在11℃的条件下生长,而最适生长温度可达到35℃[112].温度能够影响厚壁孢子的萌发和细胞分裂,Briand等[83]发现足够的温度能够促进藻体孢子萌发.其次,温度对藻丝长度也有一定影响,有研究发现,低温和高温处理下拟柱孢藻藻丝长度小于正常温度下藻丝长度,猜测可能是由于温度过高和过低会对藻细胞分裂分化活动受到影响,在形态上表现为藻丝变短[110],其具体原因仍需探讨.此外,温度也会影响CYN产量,有研究发现,拟柱孢藻最适生长温度和最大产毒量并不成正相关,例如Saker等[64]研究表明,在20℃时细胞毒性最高,而拟柱孢藻在25~30℃时有最大生长率,但该温度下藻毒素浓度较低.
Wojciechowski等[87]以生长速率和藻丝长度为指标评估了拟柱孢藻在不同光照条件下的生态生理学反应,实验结果表明,拟柱孢藻在低光照强度下的生长速率明显低于较高光照强度下的生长速率,而在低光照强度下藻丝的平均长度却明显大于高光照强度下的藻丝长度.在产毒方面,光照是否影响其产毒能力也存在争议.有研究表明光照强度、光照周期及光谱成分影响拟柱孢藻产毒能力,拟柱孢藻细胞内蛤蚌毒素(STX)浓度与光照强度呈正相关,黑暗条件下产生的毒素浓度较低,较高光强条件下细胞内STX浓度明显增高[111].而Pierangelini等[106]发现拟柱孢藻在不同光照条件下,平均胞内CYN浓度不变,表明CYN的产生不受光照条件影响.因此,为更好地理解拟柱孢藻在亚热带地区季节性暴发的成因及危害,后续研究有必要针对更多不同地区的藻株,进一步探究光照和温度条件对拟柱孢藻的生长、形态变化和产毒等的影响及其相关机理.
2.3.3 盐度的影响总体来说,拟柱孢藻适合在低盐度的条件下生长,淡水环境最适宜其生长[36],而高盐条件对拟柱孢藻的生长会产生抑制作用[112].但当水体中溶解性矿物质浓度较高时,拟柱孢藻也可在微咸水中生长[91, 113]. Moisander等[114]研究发现,当NaCl浓度为2~6 g/L时,拟柱孢藻的生长受到限制,而高于10 g/L时已不能通过固定二氧化碳进行光合作用.
2.3.4 pH的影响拟柱孢藻能够在pH较高的水体中生存.虽然pH的升高会使CO2浓度降低,但拟柱孢藻可以有效利用其他的碳源如HCO3-进行能源补充[115].在高pH值和低CO2环境下,拟柱孢藻是良好的竞争者,可成为优势藻种[116].据报道,拟柱孢藻大多在较高pH(7.0~9.6)的湖泊中出现[36, 91].
3 产毒和检测 3.1 产毒目前,已知拟柱孢藻可产生的代谢毒素主要是CYN和麻痹性贝类毒素(PSP).不同地区的拟柱孢藻产毒情况不同,有的能产生柱孢藻毒素或麻痹性贝类毒素,有的则不能.澳大利亚、新西兰、亚洲等产毒藻种以产生柱孢藻毒素为主[9, 32-34]. 1999年,人们第一次从一株巴西拟柱孢藻中鉴定出麻痹性贝类毒素[10],其中最典型的是蛤蚌毒素(saxitoxin,STX),随后又陆续从其他巴西拟柱孢藻藻种中鉴定出新蛤蚌毒素(neosaxitoxin,NSTX)和其他蛤蚌毒素类似物[117-118].截至目前,诸多学者尚未发现欧洲和北美地区藻种可产生CYN.但先前很多研究发现,某些不产CYN的欧洲和亚洲藻种也能产生一些对小鼠有生物毒性的活性代谢物[119],而这些代谢物的种类迄今为止尚不明确.
3.1.1 柱孢藻毒素CYN是一种肝毒素,1992年Ohtani等[32]首次提出CYN的化学结构. CYN(C15H21N5O7S,M=415)是一种易溶于水的多肽生物碱,具有肝毒性、细胞毒性和基因毒性[120].在水体呈电中性的条件下,CYN是一种带有正电荷和负电荷的两性离子[121].研究表明,这种两性离子可以通过阳离子交换和表面络合作用吸附到土壤及土壤矿物上[122].由于CYN存在一个三环硫酸胍两性离子组和尿嘧啶,它可在不同的热、光和pH值条件下稳定存在[123].换言之,CYN是一种耐高温、难降解的蓝藻毒素.
目前,全球范围内许多饮用水水源地中均发现了CYN的存在.值得关注的是,CYN涉及至少两种使人类中毒的疾病[124],如肠胃不适、呕吐腹泻、皮肤和眼睛刺激等. CYN可通过干扰不同的代谢途径,诱导一系列氧化应激、遗传变异、免疫抑制和肝细胞功能异常等反应[120],且对肾脏、胸腺和心脏也存在一定程度的毒性损害.目前研究指出,CYN的3个主要官能团中,只有尿嘧啶容易发生氧化反应[125].例如,CYN的尿嘧啶侧链可抑制蛋白质的翻译,并可与DNA结合引起链断裂,能够促进肿瘤的形成以及导致染色体缺失等[11, 13],但其具体的致毒机理仍在探索中.
有学者提出,某些藻种合成CYN是为了更好地适应生长环境并实现有效的繁殖扩张,即CYN是其长期进化适应环境的产物[91, 128].另有研究表明,柱孢藻毒素的产生与cyr基因簇的存在有关[129],其具体转化途径仍在探索中.产生柱孢藻毒素的藻种主要产生3种柱孢藻毒素(CYNs)即cylindrospermopsin (CYN)[10]、7-deoxy-cylindrospermopsin[128]和7-epi-cylindrospermopsin[129],结构如图 3a~c.日前,Wimmer等[130]从一株泰国拟柱孢藻中分离出两种新的柱孢藻毒素衍生物:7-deoxy-desulfo-cylindrospermopsin和7-deoxy-desulfo-12-acetylcylindrospermopsin,其结构如图 3d~e.其中CYN、deoxy-CYN占总柱孢藻毒素的95%以上,epi-CYN则微量存在,通常占比小于5%. CYN和deoxy-CYN的比例也会随藻体所处生长周期的不同发生变化[131].此外,同一地区的藻种产毒情况也有差异,Willis等[132]将从同一水库分离的24株拟柱孢藻分别同时培养,发现其生长过程中的平均胞内CYN含量及CYN与deoxy-CYN的比值各具差异.
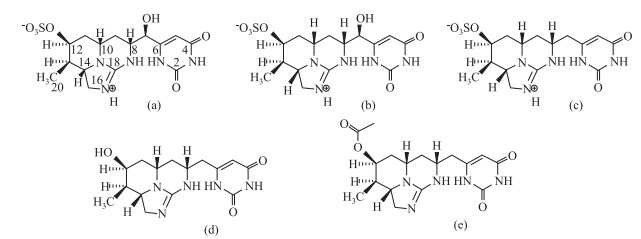
|
图 3 柱孢藻毒素及其衍生物结构[130] ((a) cylindrospermopsin; (b) 7-epi-cylindrospermopsin; (c) 7-deoxy-cylindrospermopsin; (d) 7-deoxy-desulfo-cylindrospermopsin; (e) 7-deoxy-desulfo-12-acetylcylindrospermopsin) Fig.3 The molecular structures of cylindrospermopsin and its analogs |
目前,世界卫生组织规定的CYN安全浓度限值为1 μg/L.我国没有明确规定CYN的安全浓度限值,各国提出的安全浓度参考值不等,为0.1~15 μg/L [133],如德国0.1 μg/L,澳大利亚、新西兰1 μg/L,巴西15 μg/L.然而,目前对于CYN产生生物毒性作用的机理并不明确,因此有必要开展更多研究以进一步明确CYN的致毒机理,为更有效地对水域进行风险评价和保护近水域人群健康状况提供理论支持.
目前,可以产生CYN的藻种主要是拟柱孢藻,其次还有鱼腥藻、束丝藻、尖头藻、鞘丝藻、梅崎藻等[134].此外,所有产生CYN的藻种均生活在淡水或者微咸水中,目前尚未有报道表示海水蓝藻能产生CYN,对此结果也需要展开进一步调查和研究方可确认.
3.1.2 蛤蚌毒素蛤蚌毒素(STX)是一种三环化合物(C10H17N7O4, M=299),结构如图 4.它易溶于水,在自然淡水中能够保持完整性达90 d[135],但在高温下易降解,可降解成毒性更高的变体[136].目前认为,产生STX的蓝藻主要是鱼腥藻和束丝藻,部分拟柱孢藻及鞘丝藻也可合成该毒素[137].
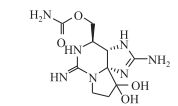
|
图 4 蛤蚌毒素结构[133] Fig.4 The molecular structure of saxitoxin |
STX是一种钠离子通道阻隔剂,同时也能够干扰钙离子和钾离子通道,影响神经细胞的轴突传导,是一种神经毒素,并可导致心输出量降低[138].自20世纪以来,STX已成为多宗中毒事件的元凶,不慎接触能导致机体麻木、瘫痪甚至死亡[139],但到目前为止,通过饮用水途径中毒尚未有记录.目前,尚没有官方准则对饮用水中STX限值作出规定,澳大利亚采用3 μg/L作为STX的安全浓度限值[140].研究表明,环境因素会对含拟柱孢藻水体中STX浓度产生一定影响,有学者提出,STX的产生可能与水的硬度和盐度有关[141].最近,Brentano等[101]发现在拟柱孢藻产生STX的过程中,水体电导率和溶解性无机氮浓度对STX浓度影响最大,并猜测可能是由于拟柱孢藻在离子应力条件下,通过分泌STX改变细胞渗透性来调节细胞的自我平衡.此外,无机氮源的增加有利于拟柱孢藻的生长,导致分泌更多的STX.
3.2 检测CYN对人体有严重危害性且能够在水体中持久存在,因此,定性定量地对CYN进行有效检测对保证水质安全以及人类健康至关重要.目前关于CYN的检测,常用以下几类方法:
3.2.1 物理化学分析高效液相色谱(HPLC)法是一种比较精确的纯化和测定毒素的方法,其分析速度快,色谱柱可重复多次使用,目前已广泛用于CYN的测定[142-143].例如,高效液相色谱-紫外分光光度法(HPLC-UV)可通过液相色谱分离CYN后,利用紫外分光光度法定量测定,研究表明,CYN在262 nm处有最大吸收波长[32, 142].在此基础上,利用HPLC-PDA(光二极管阵列)法能得到CYN的明显吸收峰,干扰较少,可比较精确地测定CYN浓度. Welker等[144]评估了利用HPLC-PDA法检测环境样品中CYN的可行性,通过对比几种不同的洗脱液,发现只有以三氟乙酸和甲醇作为洗脱液时,CYN才能被截留在C18色谱柱上,且在20 min内,用不同梯度洗脱液(5%三氟乙酸+0~50%甲酸)洗脱时,CYN相对应的峰高、峰面积和保留时间具有良好的重现性,且响应值(峰面积)与CYN浓度之间也呈现很好的线性关系.然而,用该方法检测环境样品中的CYN也存在局限性.例如,用纯水萃取CYN时,样品中存在基体干扰,可能会覆盖CYN色谱,建议在纯化过程中进一步改进.随后,Metcalf等[145]创建了一种固相提取技术(SPE),即以C18和聚石墨化碳墨盒系列(混合模式)为填料来提取检测湖水中CYN,检测限可达1 μg/L,并且填料能够实现100%再生.根据以上研究,Meriluoto和Codd等[146]提出了一种以C18-聚石墨为材料提取CYN,利用HPLC/PDA法定量测定CYN浓度的标准操作流程.此后,Wörmer等[147]又对前面的固相提取技术(SPE)进行了改进,在对样品进行酸化并加入NaCl后,仅利用石墨化碳墨盒提取CYN(二氯甲烷作为洗脱液).
另外,高效液相色谱-串联质谱(HPLC-MS/MS)同样适用于检测水体中低浓度的CYN,分析速度快,灵敏度高,已被大量采用. Eaglesham等[148]用此方法测定低浓度CYN(在1~634 μg/L范围内)时,检测到m/z 416离子([M+ H]+)到m/z 194碎片离子的转换,且结果表现出较好的线性关系(r2=1.000)和较高的准确度. Stirling和Quillia等[149]利用LC/MS和LC/MS/MS等方法选用大气压电离源和离子喷雾接口来检测水样中的CYN时,在MS/MS方法中检测到两种新的离子转换:m/z 416离子([M+H]+)到m/z 176碎片离子的转换以及m/z 433离子([M+NH4]+)到m/z 194碎片离子的转换. Guzmán-Guillén等[150]以石墨化碳墨盒作为固相萃取材料,利用HPLC-MS/MS定量检测CYN浓度,检出限为0.5 μg/L,此外,Bogialli等[151]利用LC-MS/MS分析过滤湖水中CYN浓度,得到CYN检出限为0.3 μg/L.而Kikuchi等[152]提出了一种新的LC/ESI-MS(液相色谱/电喷雾离子化-质谱)测定CYN的方法,以2-[4-(2-羟乙基)-1-哌嗪基]乙磺酸为内标物,在pH为10.5的碳酸缓冲液中提取CYN,再通过阴离子交换柱分离,实验表明该方法能有效分离和浓缩环境中低浓度的CYN且无干扰峰. Graham等[153]用反相高效液相梯度洗脱法进一步提高检测灵敏度,并用单点较准来提高定量精确度,得到CYN和7-deoxy-CYN的检出限可达0.01 μg/L.此外,黎志轩等[154]成功利用超高效液相色谱-串联质谱(UPLC-MS/MS)法对水中低浓度CYN进行检测,水样经1~3次冻融后,用Waters超高效液相色谱-串联质谱(UPLC-MS/MS)直接测定水中的柱胞藻毒素,并以416.1>194 m/z特征离子进行定量,得出该方法检出限为0.5 μg/L.
3.2.2 免疫学分析免疫分析法是一种定量测定方法,灵敏度高.酶联免疫法(ELISA)已用于检测柱孢藻毒素[155-156]及蛤蚌毒素[157-158]等,它可以通过识别并与特异性抗体结合来检测藻毒素,检测浓度范围为0.05~2.00 μg/L[121],目前,市面上可买到的主要是96微孔板Abraxis和Beacon柱胞藻毒素试剂盒,它们检测精度高,简单方便且易操作. Bláhová等[155]比较了ELISA和LC-MS两种方法对地表水中CYN的检测,结果表明两者具有很好的一致性,但用ELISA检测到的浓度要比LC-MS高,主要是由于免疫分析法不能辨别毒素变异体(如deoxy-CYN和7-epi-CYN等),同时交叉反应(即使是极少的)以及样本中其他化合物的存在也可能导致检测出的毒素浓度偏高.随后,Mohamed[159]、Zamyadi[160]和Graham[153]等也报道了利用ELISA对CYN的检测,目前,ELISA已越来越多地应用于CYN的检测研究中.
3.2.3 生物试验分析人们最早通过生物学反应检测水体中藻毒素,而小鼠体内试验是最常见的生物体内试验分析方法,主要根据不同的临床症状及半致死量揭示水体中CYN的毒性及存在情况[32, 161].除小鼠外,还有学者利用昆虫[162]、无脊椎动物(虾、水蚤、蜗牛)[163-165]、脊椎动物[166]以及植物(荠菜籽苗)[167]等进行了CYN的毒性评价研究.其中,Metcalf等[164]用盐水虾做毒性试验,发现盐水虾死亡率与CYN浓度之间呈明显的剂量效应关系,在24~72 h之间LC50(半致死浓度)值在0.71~8.1 mg/ml之间.此类体内试验方法属于半定量检测方法,灵敏度较差,所得到的毒性结果与试验生物品系有关,且易受其他因素干扰.
另外,细胞毒性检测分析技术也已用于CYN检测,该技术是对传统生物体内试验分析方法的改进,可对毒素进行精确定量.研究表明,CYN可对多种细胞产生细胞毒性,Runnegar等[168]第一次用小鼠肝细胞来检测CYN毒性,发现CYN(3.3~5.0 μmol/L)可引起小鼠肝细胞严重死亡.此后Neuman等[169]研究了HepG2(肝)、BE-2(骨髓)、Caco-2(结肠)和MNA (脑)4种细胞系对CYN及deoxyCYN的毒性反应,发现在一定浓度CYN刺激下,四种细胞系均可观察到明显的细胞形态学变化,而Caco-2细胞系对CYN和deoxyCYN最为敏感,在48 h处理下,0.25 μg/ml CYN即可对Caco-2细胞引起刺激反应.此外,Froscio等[170-171]通过一种快速无细胞蛋白质合成抑制试验来检测CYN,利用兔网织红细胞裂解液系统对CYN的检出范围为200~1200 μg/L,其结果与LC-MS/MS和HPLC-PDA检测结果有很好的一致性.
4 拟柱孢藻及柱孢藻毒素去除研究当作为饮用水水源地的湖泊或水库发生拟柱孢藻暴发时,无疑将对饮用水安全造成严重威胁,因此,对含藻水源水进行安全处理也成为当务之急.目前,水处理流程通常分为两类:一类是对水中污染物的截留(混凝、吸附、过滤等),另一类是对水中污染物的降解(氯氧化、紫外线辐射、臭氧氧化等).一方面,在含藻水处理过程中,应尽量实现藻细胞的完整无破损去除,减少胞内毒素的释放[133, 157].另一方面,对于原水中已经存在的藻毒素,也应该在后续处理中一并去除,保证饮用水安全.近年来,由于全球很多水源地均发现拟柱孢藻存在,针对拟柱孢藻及其毒素的去除也成为人们研究的热点,但目前国内外研究尚不完善.以下主要介绍近年来拟柱孢藻及其毒素的去除研究现状和展望.
4.1 拟柱孢藻去除研究 4.1.1 混凝通常饮用水处理的第一步是混凝/絮凝-沉淀,目的是去除水体中的胶体物质并降低浊度.对于藻浓度较高的水源水,一般水厂加入铁或铝盐,中和负电荷藻细胞并阻止粒子之间的静电斥力,使藻细胞凝聚,形成更大的颗粒(絮体),进而被沉降去除.该方法可有效去除藻细胞,但对水体中胞外藻毒素的去除效果不明显.
Ho等[157]用Al2(SO4)3·18H2O作为混凝剂,研究了混凝、过滤、反冲洗和含藻底藻泥堆置过程中拟柱孢藻的细胞完整性以及毒素的释放(降解)情况.研究表明混凝能有效去除拟柱孢藻,但随着混凝剂剂量的增多,胞外CYN占总CYN的比例增大,说明投加混凝剂后胞内毒素部分释放到胞外,而总CYN浓度降低,表明混凝剂对CYN有一定的吸附效果.同时,李绍秀等[172]探讨了二氧化氯氧化与混凝工艺结合去除拟柱孢藻的最佳工艺条件,在ClO2投加量0.5 mg/L,聚合氯化铝15 mg/L,ClO2与混凝剂一起投加的条件下,除藻率为98.9%.但是,利用ClO2杀灭拟柱孢藻的同时会产生甲苯等有毒副产物[173],同时ClO2能够破坏拟柱孢藻细胞,导致胞内毒素释放[156].目前,关于混凝去除水体中拟柱孢藻的研究较少,需针对不同混凝剂的混凝特性、去除效果、毒素释放情况和应用注意事项等进行深入研究,并结合其他工艺探索更高效的混凝去除方法.
4.1.2 砂滤实践证明,慢速砂滤能够同时去除蓝藻及蓝藻毒素. CYN可以通过阳离子交换和表面络合作用吸附到土壤及土壤矿物上[122],Maghsoudi等[174]发现2 h内72.6%的CYN可吸附到天然沉积物上.此外,Klitzke[175]等研究了低温、厌氧及存在溶解性有机碳(DOC)的条件下,CYN在砂质沉积物上的降解情况,表明低温厌氧不利于CYN的降解,而DOC的存在则可缩短CYN在天然沉积物上降解的延滞期.由此看出,CYN在砂质沉积物上的降解易受环境条件的制约.
另外,生物砂滤是一种用特定降解菌与砂滤结合以提高藻细胞及藻毒素去除效率的方法. Mouchet和Bonnély[176]等研究表明,用此方法可去除99%的藻细胞.由此可见,砂滤与其他处理工艺结合可能会大大提高去除效率.该方法无污染、成本低,可初步探索将其用于拟柱孢藻及CYN的去除.
4.1.3 膜处理(微滤、超滤、纳滤、反渗透)膜过滤法根据膜的孔隙大小可分为:微滤(0.1~10 μm)、超滤(1~100 nm)、纳滤(约1 nm)和反渗透(0.1 nm).该项技术对水源水中藻细胞及藻毒素的去除方面有较好的应用前景,已证明纳滤能有效去除CYN[177-178],例如Dixon等[177]研究纳滤法去除CYN,发现CYN去除效率高达90%~100%.但是纳滤和反渗透过程复杂并且成本昂贵,且滤膜堵塞和细胞破裂的问题广泛存在于现有的大部分过滤技术中,因此,该方法并没有普及应用于对藻细胞的去除.
4.2 柱孢藻毒素去除研究 4.2.1 传统方法1) 氯氧化.氯氧化法是饮用水消毒工艺中最常用的方法.它可在水中持久存在,防止病原体等污染物对饮用水的污染.各种研究表明,CYN在氯氧化过程中被迅速降解转化,去除效率高,但会伴有消毒副产物生成[160]. CYN的氯氧化降解速率受pH、温度等影响. Rodríguez等[179]发现CYN在较高pH下,更易失活,在pH为7时有最大降解速率,具有明显的二级速率常数;当温度从10℃升到30℃时,其降解速率升高2倍. Merel等[142]研究了氯氧化CYN的特性并就消毒副产物的成分进行分析,发现在20℃、pH为7的条件下,氯氧化可在2 min内降解98%以上的CYN;即使裂解尿嘧啶时伴随着3种消毒副产物形成,但副产物的毒性与CYN相比有所降低[125, 142].因此,在利用氯氧化降解CYN时,需充分考虑反应条件对其降解特性的影响,除温度、pH外,其他因素是否会对其降解产生影响有待研究.此外,人们关于氯氧化CYN的分解途径及消毒副产物种类及毒性的研究尚不充分,有待进一步完善.
2) 氯胺和二氧化氯.用二氧化氯和氯胺去除蓝藻毒素的研究并不广泛,且此方法对CYN去除效果不明显.研究显示,相同条件下,用二氧化氯降解CYN时,CYN的半衰期为14.4 h,然而用氯氧化只需要1.7 min[179].同样,氯胺作为弱氧化剂,与CYN反应缓慢,反应动力学常数较氯氧化低2400倍[180]. Cheng等[1]用氯胺和二氧化氯以标准水处理剂量去除CYN时,没有发现显著的去除效果.在杀藻方面,朱璐瑶[156]和李绍秀等[173]研究发现ClO2杀灭拟柱孢藻过程中会造成CYN释放,且伴有有机副产物生成,其产生的有机副产物种类与ClO2投加量有关:ClO2投加量越大,生成有机物的种类越少.综上所述,氯胺和二氧化氯不适合用于去除柱孢藻毒素;在杀藻应用中,应注意ClO2用量及反应时间等因素.
3) 高锰酸盐.高锰酸盐是一种强氧化剂,主要用于对水体进行预处理,以减少氯的使用和消毒副产物的形成.作为强氧化剂,高锰酸盐不产生有毒副产物[181].与其他氧化剂相比,高锰酸盐相对成本较低且易于管理,pH值适用范围宽[182],但其氧化过程周期长且会使水体呈现粉色[183].研究表明,高锰酸盐对CYN的去除效果不明显,Cheng等[1]使用剂量为360 mg·min/L的KMnO4氧化CYN时,没有发现去除效果.而KMnO4在微生物控制方面有更好的效果,有研究表明一定剂量的KMnO4能够使拟柱孢藻失活,且不会造成毒素释放[1].因此,它可以作为混凝除藻的助剂,并起到一部分预氧化(替代臭氧、二氧化氯等)作用以处理水中其他物质,达到强化混凝的效果,一般不建议单独作为主要氧化剂使用.
4) 活性炭吸附.活性炭吸附法能有效去除饮用水中的蓝藻及蓝藻毒素.使用活性炭吸附不仅不会造成藻细胞破损及其胞内毒素的释放,还可以有效去除细胞外CYN[184],但处理成本相对较高. Ho等[185]研究表明,CYN去除效果与活性炭粒径有关,粒径较小的去除效果较好,但接触时间分别为30、45和60 min时去除效果没有显著差异.目前,需要更加系统的研究来确定可有效吸附CYN的活性炭类型、剂量和接触时间等最佳条件,以提高该方法的处理效率.与此同时,活性炭也可以通过在表面或内孔固定和培养特定的微生物,随后通过生物降解去除藻毒素,可有效缓解毒素解吸困难的问题,藻毒素的清除机理也从吸附转为生物降解[186].虽然活性炭能够有效地去除藻毒素,但完全吸附去除需要大量不同的吸附剂,因此该方法需要与其他处理工艺结合而不适合作为一种单独的处理手段.
5) 生物降解.生物降解蓝藻毒素处理费用低、无二次污染.不少研究学者已发现CYN可被生物降解,且降解情况受温度、CYN初始浓度以及其他条件的影响.例如,Senogles等[186]和Smith等[187]证明CYN能够在自然水体中进行降解,并表明CYN的生物降解速率与CYN初始浓度之间近似呈线性关系,而温度和含铜灭藻剂的存在均能够影响CYN的降解[187]. Klitzke等[188]只在沉积物中观察到CYN的生物降解,并且发现对沉积物进行预处理能够增强CYN的生物降解效果.另外,Maghsoudi等[189]分别研究了湖水原水、过滤后湖水、添加藻青蛋白的滤后湖水、沉淀池污泥和滤后污泥等介质中CYN的生物降解程度,结果表明,CYN仅在沉淀池污泥中出现生物降解,12 d后CYN浓度由2.5 μg/L降到1.0 μg/L以下.由此可见,生物降解受环境限制较大,降解周期长,且在许多地区水库及底泥中并没有观察到CYN的生物降解,若能分离出CYN特定降解菌株,无疑将会大大提高CYN生物降解效率.
Mohamed等[159]首次从蓝藻水华中分离出一株CYN降解菌Bacillus(AMRI-03),该菌株在CYN存在的条件下生长良好且没有出现生长滞后期,其生长量随初始CYN浓度的增加而增大;同时,也发现CYN降解速度与CYN初始浓度有关,当CYN最高浓度为300 μg/L时,6 d即可降解完全,而浓度较低时完全降解需要7~8 d.日前,Dziga等[190]从印度某湖泊分离出一株气单胞菌,并证明该菌株可有效降解CYN,生物降解效果取决于pH和温度.此外,何雅孜等[191]分离筛选的一株溶藻细菌L7能够显著抑制某富营养化水体中拟柱孢藻(优势种)的生长.因此,发现、分离更多CYN降解菌和拟柱孢藻溶藻菌对高效降解CYN及抑制拟柱孢藻的生长有重要意义.
4.2.2 高级氧化法羟基自由基(OH·)具有强氧化性,与底物反应迅速,几乎可以氧化所有的有机化合物.高级氧化法采用OH·作为主要氧化剂可高效去除饮用水中多种毒素及其他污染物,已经受到诸多研究人员的广泛关注.
1) 光催化氧化.紫外辐射可以生成高活性的OH·,已成为一项具备广泛应用前景的饮用水消毒手段,使用波长在240~280 nm处的紫外光诱导水体中微生物失活以优化水质.研究表明,光催化氧化可以有效去除CYN,其中对降解起主要作用的是OH·[192-195].目前研究较多的集中在TiO2光催化氧化.实验表明,利用TiO2光催化氧化可提高CYN的降解效率,例如Senogles等[196]发现,单独紫外辐射时,降解CYN的半衰期为14 min,而用TiO2光催化氧化可缩短至2 min.此外,光催化的处理效果取决于照射灯的类型和设计、光辐照强度、毒素的初始浓度以及水的浊度、pH等. Chen等[194]研究表明随着光照强度的增强,CYN降解速率升高,而CYN初始浓度和pH的升高则使降解速率降低.总体来说,光催化氧化是一种有效去除CYN的方法,但今后应加强对其潜在的消毒副产物的研究.
| 表 2 拟柱孢藻及其毒素的去除方法比较 Tab.2 The comparison of removal methods on C. raciborskii and CYN |
其次,UV或可见光辅助芬顿氧化(即光-芬顿氧化)可提高藻毒素的降解速率,且可以克服芬顿试剂只在酸性环境下能有效降解藻毒素的局限[197].目前,光-芬顿氧化法去除CYN的研究鲜有报道,有必要探索这种高级氧化法对CYN的去除效果、降解条件和反应途径等以提高对该方法去除CYN的认识.
2) 臭氧氧化.臭氧是一种在水处理过程中应用最普遍的氧化剂之一,可有效去除CYN[1, 180, 198],且对CYN的去除效率要高于氯氧化和高锰酸盐氧化[180],但尚未考虑其副产物和残余毒性.臭氧可用于混凝前或混凝后,但在混凝前投加臭氧可能会造成细胞膜破裂,引起胞内毒素的释放[199],因此不建议在混凝前投加.由于臭氧不能在水中持久存在,又经常被用于除去水中微量有机物,因此,臭氧可以和H2O2或Fe(Ⅱ)联用,产生更多OH·,提高对微量藻毒素的降解能力.目前,利用臭氧和O3/H2O2或O3/Fe(Ⅱ)联用去除藻毒素并没有得到广泛研究.初步研究显示,O3/H2O2或O3/Fe(Ⅱ)联用与单独O3相比,都可强化微囊藻毒素MCs和类毒素ANTX-a的降解[199-200].因此可以进行进一步研究O3/H2O2或O3/Fe(Ⅱ)联用是否可以增强CYN的降解.
5 结论与展望近20年来,拟柱孢藻在全球分布范围不断扩大,逐渐由热带、亚热带地区向温带地区扩散.由于其对光照和温度的适应范围广,并可有效利用环境中不同形式的氮磷,在许多淡水水生系统中,拟柱孢藻都是良好的竞争者,在非最适生长环境中,仍可持续生长,甚至成为优势藻种.在全球温室效应加剧的背景下,其良好的环境适应性有助于其生长区域的急速扩张.
拟柱孢藻可产生柱孢藻毒素和蛤蚌毒素等对人体健康有害的毒素.因此,在研究过程中,除了分离、培养拟柱孢藻并探索其生长条件和营养特性外,还需要调查其毒素在天然水体中的分布情况并分析该毒素的代谢机理,以进一步控制该毒素对人类及环境的危害.就此而言,定性定量检测其代谢毒素也显得十分重要.在现有检测技术中,LC-MS/MS可定量测定CYN及其类似物;酶联免疫检测(ELISA)操作简单,检测精度高,也具有较好的应用前景.
其次,拟柱孢藻及柱孢藻毒素在水源水中的存在严重威胁人类的饮水安全,在被该藻污染的水源水进入供水系统之前,需完整去除藻细胞,并最大程度实现藻毒素的降解.目前,臭氧、光催化氧化等高级氧化技术可高效降解柱孢藻毒素.
近年来,对拟柱孢藻的研究日益增多,但仍有许多方面尚未探索,今后有必要在以下方面开展研究:
1) 在拟柱孢藻的分布和扩张方面,随着全球气候变暖程度的加深,拟柱孢藻可能继续向其他地区扩张,有必要对不同地区水域进行跟踪检测报道,对该藻在湖泊或水库中的出现应引起重视.此外,人们正通过对不同区域藻种进行基因数据分析,推断其亲缘相似性,但其扩张的具体原因还需进一步深入探究.
2) 拟柱孢藻的形态差异较大,营养元素和环境因子对其形态变化及产毒过程有重要影响.因此,一方面,需进一步了解营养元素的变化对其生长和产毒过程的影响.例如,除氮磷外,有必要针对其他元素(如Fe、S元素)对拟柱孢藻生长和产毒过程的影响以及相关机理展开研究.另一方面,温度、光照、盐度、pH等环境因子对拟柱孢藻生态生理特征的影响仍在探索中,可针对不同纬度地区的藻种,进一步探究温度、光照等环境因子对其生长的影响及其相关机理.
3) 在拟柱孢藻的产毒过程及其代谢物毒性方面,需进一步研究CYN、STX的产生与其相关基因表达的关系以及具体的产毒途径,此外,CYN、STX对生物体的致毒机理也需进一步探究.目前,对某些拟柱孢藻有毒代谢物尚未做出具体鉴定,后续应加大对此类代谢物的鉴定和致毒机理的研究,为进一步预测和评价其毒性以及对人类健康的影响提供依据,并制定相关的有毒代谢物安全浓度限值.
4) 自CYN发现以来,各国学者研究和发展了诸多此类毒素的测定方法,由于柱胞藻毒素存在异构体,在实际应用中,使得一些方法存在一定的局限性(如ELISA),或某些方法存在操作复杂,灵敏度不够等缺点(如LC-UV),因此,研究并开发更多精确简便的测定方法对CYN的检测以及拟柱孢藻的后续研究有重要意义.
5) 在拟柱孢藻及其毒素的去除方面,关于混凝法仍需进一步研究不同混凝剂的混凝特性、去除效果、毒素释放情况和应用注意事项等.生物降解法有较广阔的应用前景,发现并分离更多拟柱孢藻溶藻菌及CYN降解菌并探索适宜的生物降解条件对拟柱孢藻及其毒素的去除也具有重要意义.其次,为高效去除CYN并控制消毒副产物的生成,需进一步探究高级氧化法对CYN的降解条件、反应途径及副产物形成情况,并可通过对多种处理方法的有效结合达到高效除藻和减少消毒副产物的目的.例如,光-芬顿氧化法、O3/H2O2或O3/Fe(Ⅱ)联用法等是否可以增强CYN的降解需进一步探索.此外,更多兼具良好经济实用性和实践性的先进去除工艺也需要大量开发和研究.
| [1] |
Cheng Xiaoliang, Shi Honglan, Adams CD et al. Effects of oxidative and physical treatments on inactivation of Cylindrospermopsis raciborskii and removal of cylindrospermopsin. Water Science and Technology, 2009, 60(3): 689-697. DOI:10.2166/wst.2009.385 |
| [2] |
Paerl HW, Rd FR, Moisander PH et al. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Scientific World Journal, 2001, 1(1): 76-113. |
| [3] |
Antunes JT, Leão PN, Vasconcelos VM. Cylindrospermopsis raciborskii: Review of the distribution, phylogeography, and ecophysiology of a global invasive species. Frontiers in Microbiology, 2014, 473(6): 473. |
| [4] |
Haande S, Rohrlack T, Ballot A et al. Genetic characterisation of Cylindrospermopsis raciborskii, (Nostocales, Cyanobacteria) isolates from Africa and Europe. Harmful Algae, 2008, 7(5): 692-701. DOI:10.1016/j.hal.2008.02.010 |
| [5] |
Sinha R, Pearson LA, Davis TW et al. Increased incidence of Cylindrospermopsis raciborskii in temperate zones—Is climate change responsible?. Water Research, 2012, 46(5): 1408-1419. DOI:10.1016/j.watres.2011.12.019 |
| [6] |
Hamilton PB, Ley LM, Dean S et al. The occurrence of the cyanobacterium Cylindrospermopsis raciborskii in Constance Lake: An exotic cyanoprokaryote new to Canada. Phycologia, 2005, 44(1): 17-25. DOI:10.2216/0031-8884(2005)44[17:TOOTCC]2.0.CO;2 |
| [7] |
Han Boping, Lin Guihua, Zhong Xiuying eds. Distribution and detection of cyanobacteria and cyanobacterial toxins in reservoir: The research of typical water supply reservoir in Guangdong Province. Beijing: China Environmental Science Press, 2006. [韩博平, 林桂花, 钟秀英. 水库蓝藻和蓝藻毒素分布与检测:广东省典型供水水库研究. 北京: 中国环境科学出版社, 2006.]
|
| [8] |
Bourke ATC, Hawes RB, Neilson A et al. An outbreak of hepato-enteritis (the Palm Island mystery disease) possibly caused by algal intoxication. Toxicon, 1983, 21(3): 45-48. |
| [9] |
Hawkins PR, Runnegar MT, Jackson AR et al. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Wołoszyńska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Applied & Environmental Microbiology, 1985, 50(5): 1292-1295. |
| [10] |
Lagos N, Onodera H, Zagatto PA et al. The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil. Toxicon, 1999, 37(10): 1359-1373. DOI:10.1016/S0041-0101(99)00080-X |
| [11] |
Humpage AR, Fenech M, Thomas P et al. Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin, cylindrospermopsin. Mutation Research/fundamental & Molecular Mechanisms of Mutagenesis, 2001, 472(1/2): 155-61. |
| [12] |
Shen X, Lam PKS, Shaw GR et al. Genotoxicity investigation of a cyanobacterial toxin, cylindrospermopsin. Toxicon, 2002, 40(10): 1499-1501. DOI:10.1016/S0041-0101(02)00151-4 |
| [13] |
Froscio SM, Humpage AR, Burcham PC et al. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environmental Toxicology, 2003, 18(4): 243-251. DOI:10.1002/(ISSN)1522-7278 |
| [14] |
O'neil JM, Davis TW, Burford MA et al. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae, 2012, 14: 313-334. DOI:10.1016/j.hal.2011.10.027 |
| [15] |
Moreira C, Fathalli A, Vasconcelos V et al. Phylogeny and biogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Archives of Microbiology, 2015, 197(1): 47-52. DOI:10.1007/s00203-014-1052-5 |
| [16] |
Wołoszyńska J. Das phytoplankton einiger javanischer Seen, mit Berücksichtigung des Sawa-Planktons. Imprimerie de l'Université, 1912.
|
| [17] |
Singh RN. Seasonal variants of Anabaenopsis raciborskii Wołosz. Hydrobiologia, 1962, 20(1): 87-91. DOI:10.1007/BF00038737 |
| [18] |
Chen Aomi. The evaluation of nutrition status and the risk of cyanobacterial bloom of 33 large and medium-sized reservoirs in Jiangmen City. Guangdong Water Conservancy and Hydropower, 2013(7): 51-54. [陈奥密. 江门市33宗大中型水库营养状态评价及蓝藻水华风险评估. 广东水利水电, 2013(7): 51-54.] |
| [19] |
Jiang Qiming, Hou Wei, Gu Jiguang et al. Nutritional status and population characteristics of Cyanobacteria in small and medium sized reservoirs in Guangzhou, southern China. Ecology and Environment, 2010, 19(10): 2461-2467. [江启明, 侯伟, 顾继光等. 广州市典型中小型水库营养状态与蓝藻种群特征. 生态环境学报, 2010, 19(10): 2461-2467. DOI:10.3969/j.issn.1674-5906.2010.10.035] |
| [20] |
Liu Lei, Lei Lamei, Xiao Lijuan et al. Dynamics of the trophic state and phytoplankton community of a small reservoir in South China. Ecological Science, 2008, 27: 71-76. [刘蕾, 雷腊梅, 肖利娟等. 一座南亚热带小型水库水体营养状态与浮游植物的季节变化. 生态科学, 2008, 27(2): 71-76.] |
| [21] |
Wang Xiaohui. Structure characteristics of phytoplankton community in Dashuiqiao Reservoir. Journal of Hydroecology, 2013, 34(2): 40-45. [王晓辉. 大水桥水库浮游植物群落结构特征研究. 水生态学杂志, 2013, 34(2): 40-45.] |
| [22] |
Zou Hongjü, Hu Ren, Han Boping. Structure and dynamics of phytoplankton community in Hedi Reservoir, South China. Journal of Tropical and Subtropical Botany, 2010, 18(2): 196-202. [邹红菊, 胡韧, 韩博平. 鹤地水库浮游植物群落的结构与动态. 热带亚热带植物学报, 2010, 18(2): 196-202.] |
| [23] |
Lei L, Peng L, Huang X et al. Occurrence and dominance of Cylindrospermopsis raciborskii and dissolved cylindrospermopsin in urban reservoirs used for drinking water supply, South China. Environmental Monitoring and Assessment, 2014, 186(5): 3079-3090. DOI:10.1007/s10661-013-3602-8 |
| [24] |
Zha Guangfang. Investigation on the ecological factor in Cylindrospermopsis raciborskii bloom in low salty prawn ponds. Ecological Science, 2009(4): 293-298. [查广方. 虾池拟柱孢藻爆发的生态因子调查. 生态科学, 2009(4): 293-298.] |
| [25] |
Zheng Hongping. Phytoplankton community characteristics and nutrition analysis in Dongxun Reservoir. Chemical Engineering & Equipment, 2012(5): 193-195. [郑洪萍. 东圳水库浮游植物群落特征与营养状况分析. 化学工程与装备, 2012(5): 193-195.] |
| [26] |
Li Qiuhua, Shang Lihai, Li Guanghui et al. Temporal and spatial characteristics of phytoplankton community in Wanfeng Reservoir. Chinese Journal of Ecology, 2011, 30(5): 1031-1038. [李秋华, 商立海, 李广辉等. 万峰湖浮游植物群落的时空分布. 生态学杂志, 2011, 30(5): 1031-1038.] |
| [27] |
Chen Lili, Li Qiuhua, Teng Mingde et al. Cyanobacteria composition and microcystins distribution of Wanfeng Reservoir and Baihua Reservoir on Guizhou Plateau. Ecology and Environment, 2011, 20(6): 1068-1074. [陈丽丽, 李秋华, 滕明德等. 两座高原水库蓝藻群落结构与微囊藻毒素的分布对比研究. 生态环境学报, 2011, 20(6): 1068-1074.] |
| [28] |
Li R, Carmichael WW, Brittain S et al. First report of the cyanotoxins cylindrospermopsin and deoxycylindrospermopsin from Raphidiopsis curvata (Cyanobacteria). Journal of Phycology, 2001, 37(6): 1121-1126. DOI:10.1111/jpy.2001.37.issue-6 |
| [29] |
Jiang Y, Xiao P, Yu G et al. Sporadic distribution and distinctive variations of cylindrospermopsin genes in cyanobacterial strains and environmental samples from Chinese freshwater bodies. Applied & Environmental Microbiology, 2014, 80(17): 5219-5230. |
| [30] |
Yamamoto Y, Shiah FK, Hsu SC. Seasonal variation in the net growth rate of the cyanobacterium Cylindrospermopsis raciborskii in a shallow artificial pond in northern Taiwan. Plankton and Benthos Research, 2013, 8(2): 68-73. DOI:10.3800/pbr.8.68 |
| [31] |
Wu Donghao, Xu Zhaoan, Wang Yu et al. Seasonal variation of phytoplankton community structure in Hengshan Reservoir. Journal of Hydroecology, 2012, 33(4): 54-57. [吴东浩, 徐兆安, 王玉等. 横山水库浮游植物群落结构季节性变化特征. 水生态学杂志, 2012, 33(4): 54-57.] |
| [32] |
Ohtani I, Moore RE, Runnegar MT. Cylindrospermopsin: A potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. Journal of the American Chemical Society, 1992, 114(20): 7941-7942. DOI:10.1021/ja00046a067 |
| [33] |
Wood SA, Stirling DJ. First identification of the cylindrospermopsin—Producing cyanobacterium Cylindrospermopsis raciborskii in New Zealand. New Zealand Journal of Marine and Freshwater Research, 2003, 37(4): 821-828. DOI:10.1080/00288330.2003.9517211 |
| [34] |
Li R, Carmichael WW, Brittain S et al. Isolation and identification of the cyanotoxin cylindrospermopsin and deoxy-cylindrospermopsin from a Thailand strain of Cylindrospermopsis raciborskii(Cyanobacteria). Toxicon, 2001, 39(7): 973-980. DOI:10.1016/S0041-0101(00)00236-1 |
| [35] |
Mohamed ZA, Al-Shehri AM. Assessment of cylindrospermopsin toxin in an arid Saudi lake containing dense cyanobacterial bloom. Environmental Monitoring & Assessment, 2013, 185(3): 2157-2166. |
| [36] |
Padisák J. Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju, an expanding, highly adaptive cyanobacterium: Worldwide distribution and review of its ecology. Archiv für Hydrobiologie Supplementband Monographische Beitrage, 1997, 107(4): 563-593. |
| [37] |
Dyble J, Paerl HW, Neilan BA. Genetic characterization of Cylindrospermopsis raciborskii (cyanobacteria) isolates from diverse geographic origins based on nifH and cpcBA-IGS nucleotide sequence analysis. Applied & Environmental Microbiology, 2002, 68(5): 2567-2571. |
| [38] |
Gugger M, Molica R, Le BB et al. Genetic diversity of Cylindrospermopsis strains (cyanobacteria) isolated from four continents. Applied & Environmental Microbiology, 2005, 71(2): 1097-1100. |
| [39] |
Skuja H. Süβwasseralgen aus Griechenland und Kleinasien. Hedwigia, 1937, 77: 15-73. |
| [40] |
Prescott GW, Andrews TF. A new species of anabaenopsis in a Kansas lake with notes on limnology. Hydrobiologia, 1955, 7(1): 60-63. |
| [41] |
Kling H. Variation in six planktonic cyanophyte genera in Lake Victoria (East Africa). Algological Studies/Archiv für Hydrobiologie, 1991, Supplement Volumes: 21-45.
|
| [42] |
Kutsharova M. Flora vodoroslei risovih polei dolini r. Tsirtsik I ee znatsenie—Thesis Tashkent(in Russian). 1963.
|
| [43] |
Imevbore AMA. Hydrology and Plankton of Eleiyele Reservoir Ibadan, Nigeria. Hydrobiologia, 1967, 30(30): 154-176. |
| [44] |
Gupta RS, Kumar HD. Blue-green algal flora of Udaipur and its neighbourhood. Revista Algological, 1968, 2: 9-103. |
| [45] |
Hortobágyi T. Phytoplankton organisms from three reservoirs on the Jamuna River, India. India Studia Biologica Hungaricae, 1969, 8: 1-80. |
| [46] |
Ergashev AEK. Characteristike algoflori farhadskogo vodohranulisha na r. Sir-Daria. (Data to algal flora of the Farhad reservoir on river Sir-Daria). In:Flora vodoeoslei vodoemov Uzbeghistana (Algal Flora of the Reservoirs in Uzbeghistan) Tashkent (in Russian). 1969.
|
| [47] |
Hill H. Anabaenopsis raciborskii Wołoszyńska in Minnesota lakes. Journal of the Minnesota Academy of Sciences, 1970, 36: 80-82. |
| [48] |
Vinogradska T. Pro posirenniya ta ekologiyu ridkishih ta tsikavih vidib sinozelenih vodorostei: On distribution and ecocoly of rare and interesting species of blue-green algae. Ukrainskiy Botanical, 1974, 31: 733-739. |
| [49] |
Lewis WM, Wiebezahn FH. Chemistry, energy flow, and community structure in some Venezuelan fresh waters. Arch Hydrobiologia, 1976, 50: 145-207. DOI:10.1007/BF00019817 |
| [50] |
Horecka, Komarek. Taxonomic position of three planktonic blue-green algae from the genera Aphanizomenon and Cylindrospermopsis. Preslia, 1979, 51: 289-312. |
| [51] |
Oláh J, Samra M, Moneim HAA et al. Nitrogen fixation in fish-producing agro-ecosystems, Lake Balaton, reservoir, fishponds. A Halhustermeles Fejlesztese, 1981, 10: 1-8. |
| [52] |
Rott E. A contribution to the phytoplankton species composition of Parakrama Samudra, an ancient man-made lake in Sri Lanka. Netherlands: Springer, 1983, 209-226.
|
| [53] |
Komárek J. Sobre las cianofíceas de Cuba:(3) Especies planctónicas que forman florecimientos de las aguas. Acta Botanica Cubana, 1984, 19: 1-33. |
| [54] |
Lind OT. Patterns of phytoplankton populations and their relationship to trophic state in an elongate reservoir. Verhandlung Internationale Vereinigung Limnologie, 1984, 22(3). |
| [55] |
Kalff J, Watson. Phytoplankton and its dynamics in two tropical lakes: A tropical and temperate zone comparison. Hydrobiologia, 1986, 138(1): 161-176. DOI:10.1007/BF00027238 |
| [56] |
Hecky RE, Kling HJ. Phytoplankton ecology of the great lakes in the rift valleys of Central Africa. Archiv für Hydrobiologe Beiheft Ergebnisse der Limnologie, 1987, 25: 197-228. |
| [57] |
Romo S, Miracle MR. Population dynamics and ecology of subdominant phytoplankton species in a shallow hypertrophic lake (Albufera of Valencia, Spain). Hydrobiologia, 1994, 273(1): 37-56. DOI:10.1007/BF00126767 |
| [58] |
Hooker EL, Chow N, Saavedra R. Phytoplankton biomass and primary productivity of Lake Masaya (Nicaragua). Internationale Vereinigung fur Theoretische und Angewandte Limnologie Verhandlungen, 1993, 25(2). |
| [59] |
Branco CWC, Senna PAC. Factors influencing the development of Cylindrospermopsis raciborskii and Microcystis aeruginosa in the Paranoá Reservoir, Brasília, Brazil. Algological Studies/Archiv für Hydrobiologie, 1994, Supplement Volumes: 85-96. |
| [60] |
Harris GP, Baxter G. Interannual variability in phytoplankton biomass and species composition in a subtropical reservoir. Freshwater Biology, 1996, 35(3): 545-560. |
| [61] |
Dokulil MT, Mayer J. Population dynamics and photosynthetic rates of a Cylindrospermopsis-Limnothrix association in a highly eutrophic urban lake, Alte Donau, Vienna, Austria. Algological Studies/Archiv für Hydrobiologie, 1996, Supplement Volumes: 179-195. |
| [62] |
Krienitz L, Hegewald E. Über das Vorkommen von wärmeliebenden Blaualgenarten in einem norddeutschen See. Lauterbornia, 1996, 26: 55-63. |
| [63] |
Harding WR. The phytoplankton ecology of a hypertrophic, shallow lake, with particular reference to primary production, periodicity and diversity. Publisher not identified, 1996.
|
| [64] |
Saker ML, Griffiths DJ. The effect of temperature on growth and cylindrospermopsin content of seven isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from water bodies in northern Australia. Phycologia, 2000, 39(39): 349-354. |
| [65] |
Zohary T. Changes to the phytoplankton assemblage of Lake Kinneret after decades of a predictable, repetitive pattern. Freshwater Biology, 2004, 49(10): 1355-1371. DOI:10.1111/fwb.2004.49.issue-10 |
| [66] |
Bouaïcha N, Nasri AB. First report of cyanobacterium Cylindrospermopsis raciborskii, from Algerian freshwaters. Environmental Toxicology, 2004, 19(5): 541-543. DOI:10.1002/tox.v19:5 |
| [67] |
Mohamed ZA. First report of toxic Cylindrospermopsis raciborskii, and Raphidiopsis mediterranea, (Cyanoprokaryota) in Egyptian fresh waters. FEMS Microbiology Ecology, 2007, 59(3): 749-761. DOI:10.1111/fem.2007.59.issue-3 |
| [68] |
Chonudomkul D, Yongmanitchai W, Theeragool G et al. Morphology, genetic diversity, temperature tolerance and toxicity of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) strains from Thailand and Japan. FEMS Microbiology Ecology, 2004, 48(3): 345-355. DOI:10.1016/j.femsec.2004.02.014 |
| [69] |
Gugger MF, Hoffmann L. Polyphyly of true branching cyanobacteria (Stigonematales). International Journal of Systematic and Evolutionary Microbiology, 2004, 54(2): 349-357. DOI:10.1099/ijs.0.02744-0 |
| [70] |
Neilan BA, Saker ML, Fastner J et al. Phylogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Molecular Ecology, 2003, 12(1): 133-140. |
| [71] |
Jones WW, Sauter S. Distribution and abundance of Cylindrospermopsis raciborskii in Indiana lakes and reservoirs. Prepared for Office of Water Quality. Indiana Depart. of Environ. Man. Indianapolis, IN, 2005.
|
| [72] |
Hong Y, Steinman A, Biddanda B et al. Occurrence of the toxin-producing cyanobacterium Cylindrospermopsis raciborskii in Mona and Muskegon Lakes, Michigan. Journal of Great Lakes Research, 2006, 32(3): 645-652. DOI:10.3394/0380-1330(2006)32[645:OOTTCC]2.0.CO;2 |
| [73] |
Messineo V, Melchiorre S, Corcia AD et al. Seasonal succession of Cylindrospermopsis raciborskii, and Aphanizomenon ovalisporum, blooms with cylindrospermopsin occurrence in the volcanic Lake Albano, Central Italy. Environmental Toxicology, 2010, 25(1): 18-27. |
| [74] |
Fathalli A, Jenhani ABR, Moreira C et al. First observation of the potentially toxic and invasive cyanobacterium species Cylindrospermopsis raciborskii (Woloszynska) in Tunisian freshwaters: Toxicity assessment and molecular characterization. Fresenius Environmental Bulletin, 2010, 19(6): 1074-1083. |
| [75] |
Alster A, Kaplan-Levy RN, Sukenik A et al. Morphology and phylogeny of a non-toxic invasive Cylindrospermopsis raciborskii, from a Mediterranean Lake. Hydrobiologia, 2010, 639(1): 115-128. DOI:10.1007/s10750-009-0044-y |
| [76] |
Figueredo CC, Giani A. Phytoplankton community in the tropical lake of Lagoa Santa (Brazil): Conditions favoring a persistent bloom of Cylindrospermopsis raciborskii. Limnologica-Ecology and Management of Inland Waters, 2009, 39(4): 264-272. DOI:10.1016/j.limno.2009.06.009 |
| [77] |
Cvijan M, Fužinato S. The first finding of Cylindrospermopsis raciborskii (Woloszińska) Seenayya et Subba Raju, 1972 (Cyanoprokaryota) in Serbia. Archives of Biological Sciences, 2011, 63(2): 507-510. DOI:10.2298/ABS1102507C |
| [78] |
Vidal L, Kruk C. Cylindrospermopsis raciborskii (Cyanobacteria) extends its distribution to Latitude 34°53'S: Taxonomical and ecological features in Uruguayan eutrophic lakes. Pan-American Journal of Aquatic Sciences, 2008, 3(2): 142-151. |
| [79] |
Kokociński M, Stefaniak K, Mankiewicz-Boczek J et al. The ecology of the invasive cyanobacterium Cylindrospermopsis raciborskii (Nostocales, Cyanophyta) in two hypereutrophic lakes dominated by Planktothrix agardhii(Oscillatoriales, Cyanophyta). European Journal of Phycology, 2010, 45(4): 365-374. DOI:10.1080/09670262.2010.492916 |
| [80] |
Kokociński M, Dziga D, Spoof L et al. First report of the cyanobacterial toxin cylindrospermopsin in the shallow, eutrophic lakes of western Poland. Chemosphere, 2009, 74(5): 669-675. DOI:10.1016/j.chemosphere.2008.10.027 |
| [81] |
Wu JH, Hsu MH, Hung CH et al. Development of a hierarchical oligonucleotide primer extension assay for the qualitative and quantitative analysis of Cylindrospermopsis raciborskii subspecies in freshwater. Microbes & Environments, 2010, 25(2): 103-110. |
| [82] |
Yilmaz M, Phlips EJ. Diversity of and selection acting on cylindrospermopsin cyrB gene adenylation domain sequences in Florida. Applied & Environmental Microbiology, 2011, 77(7): 2502-2507. |
| [83] |
Dao TS, Cronberg G, Nimptsch J et al. Toxic cyanobacteria from Tri An Reservoir, Vietnam. Nova Hedwigia, 2010, 90(3/4): 433-448. |
| [84] |
Tundisi JG, Matsumura-Tundisi T, Tundisi J et al. A bloom of cyanobacteria (Cylindrospermopsis raciborskii) in UHE Carlos Botelho (Lobo/Broa) reservoir: A consequence of global change?. Brazilian Journal of Biology, 2015, 75(2): 507-508. DOI:10.1590/1519-6984.24914 |
| [85] |
Nimptsch J, Woelfl S, Osorio S et al. First record of toxins associated with cyanobacterial blooms in oligotrophic North Patagonian lakes of Chile-A genomic approach. International Review of Hydrobiology, 2015, 101(1): 11-20. |
| [86] |
Seenayya G, Raju NS. On the ecology and systematic position of the alga known as Anabaenopsis raciborskii (Wolosz.) Elenk. and a critical evaluation of the forms described under the genus Anabaenopis. 1972.
|
| [87] |
Wojciechowski J, Fernandes LF, Fonseca FVB. Morpho-physiological responses of a subtropical strain of Cylindrospermopsis raciborskii (Cyanobacteria) to different light intensities. Acta Botanica Brasilica, 2016(AHEAD): 0-0. |
| [88] |
Fu Changning. Improvement of Cylindrospermopsis raciborskii detection. Straits Science, 2012(7): 38-40. [傅昶宁. 拟柱孢藻检测技术改进. 海峡科学, 2012(7): 38-40.] |
| [89] |
Bittencourt-Oliveira MC, Buch B, Hereman TC et al. Effects of light intensity and temperature on Cylindrospermopsis raciborskii (Cyanobacteria) with straight and coiled trichomes: Growth rate and morphology. Brazilian Journal of Biology, 2012, 72(2): 343-351. DOI:10.1590/S1519-69842012000200016 |
| [90] |
Plominsky ÁM, Delherbe N, Mandakovic D et al. Intercellular transfer along the trichomes of the invasive terminal heterocyst forming cyanobacterium Cylindrospermopsis raciborskii CS-505. FEMS Microbiology Letters, 2015, 362(5): u9. |
| [91] |
Briand JF, Robillot C, Quiblier-Lloberas C et al. Environmental context of Cylindrospermopsis raciborskii (Cyanobacteria) blooms in a shallow pond in France. Water Research, 2002, 36(13): 3183-3192. DOI:10.1016/S0043-1354(02)00016-7 |
| [92] |
McGregor GB, Fabbro LD. Dominance of Cylindrospermopsis raciborskii (Nostocales, Cyanoprokaryota) in Queensland tropicaland subtropical reservoirs: Implications for monitoring and management. Lakes & Reservoirs: Research & Management, 2000, 5(3): 195-205. |
| [93] |
Recknagel F, Orr PT, Cao H. Inductive reasoning and forecasting of population dynamics of Cylindrospermopsis raciborskii in three sub-tropical reservoirs by evolutionary computation. Harmful Algae, 2014, 31(1): 26-34. |
| [94] |
Antenucci JP, Ghadouani A, Burford MA et al. The long-term effect of artificial destratification on phytoplankton species composition in a subtropical reservoir. Freshwater Biology, 2005, 50(50): 1081-1093. |
| [95] |
Burford MA, Johnson SA, Cook AJ et al. Correlations between watershed and reservoir characteristics, and algal blooms in subtropical reservoirs. Water Research, 2007, 41(18): 4105-4114. DOI:10.1016/j.watres.2007.05.053 |
| [96] |
Présing M, Herodek S, Vörös L et al. Nitrogen fixation, ammonium and nitrate uptake during a bloom of Cylindrospermopsis raciborskii in Lake Balaton. Archiv für Hydrobiologie, 1996, 136(4): 553-562. |
| [97] |
Kenesi G, Shafik HM, Kovács AW et al. Effect of nitrogen forms on growth, cell composition and N2 fixation of Cylindrospermopsis raciborskii in phosphorus-limited chemostat cultures. Hydrobiologia, 2009, 623(1): 191-202. DOI:10.1007/s10750-008-9657-9 |
| [98] |
Saker ML, Neilan BA. Varied diazotrophies, morphologies, and toxicities of genetically similar isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from Northern Australia. Applied & Environmental Microbiology, 2001, 67(4): 1839-1845. |
| [99] |
Ammar M, Comte K. Initial growth phases of two bloom-forming cyanobacteria (Cylindrospermopsis raciborskii and Planktothrix agardhii) in monocultures and mixed cultures depending on light and nutrient conditions. Annales de Limnologie-International Journal of Limnology, 2014, 50(3): 231-240. DOI:10.1051/limn/2014096 |
| [100] |
Saker ML, Neilan BA, Griffiths DJ. Two morphological forms of Cylindrospermopsis raciborskii (Cyanobacteria) isolated from Solomon Dam, Palm Island, Queensland. Journal of Phycology, 1999, 35(3): 599-606. DOI:10.1046/j.1529-8817.1999.3530599.x |
| [101] |
Brentano DM, Giehl ELH, Petrucio MM. Abiotic variables affect STX concentration in a meso-oligotrophic subtropical coastal lake dominated by Cylindrospermopsis raciborskii, (Cyanophyceae). Harmful Algae, 2016, 56: 22-28. DOI:10.1016/j.hal.2016.03.017 |
| [102] |
Willis A, Adams MP, Chuang AW et al. Constitutive toxin production under various nitrogen and phosphorus regimes of three ecotypes of Cylindrospermopsis raciborskii, ((Wołoszyńska) Seenayya et Subba Raju). Harmful Algae, 2015, 47: 27-34. DOI:10.1016/j.hal.2015.05.011 |
| [103] |
Wiedner C, Rücker J, Fastner J et al. Seasonal dynamics of cylindrospermopsin and cyanobacteria in two German lakes. Toxicon, 2008, 52(6): 677-686. DOI:10.1016/j.toxicon.2008.07.017 |
| [104] |
Dai JingJun, Peng Liang, Yu Ting et al. The effects of phosphorus and nitrogen on the growth of Cylindrospermopsis raciborskii N8 isolated from the Zhenhai Reservoir. Acta Hydrobiologica Sinica, 2015, 39(3): 533-539. [戴景峻, 彭亮, 于婷等. 镇海水库拟柱孢藻的分离鉴定和氮磷对其生长的影响. 水生生物学报, 2015, 39(3): 533-539. DOI:10.7541/2015.70] |
| [105] |
Ye Linlin, Zhang Min, Kong Fanxiang et al. Progress and prospect of research on cyanobacteria nitrogen fixing in aquatic ecosystem. J Lake Sci, 2014, 26(1): 9-18. [叶琳琳, 张民, 孔繁翔等. 水生生态系统蓝藻固氮作用研究进展与展望. 湖泊科学, 2014, 26(1): 9-18. DOI:10.18307/2014.0102] |
| [106] |
Pierangelini M, Sinha R, Willis A et al. Constitutive cylindrospermopsin pool size in Cylindrospermopsis raciborskii under different light and CO2 partial pressure conditions. Applied & Environmental Microbiology, 2015, 81(9): 3069-3076. |
| [107] |
Yu Ting, Dai Jingjun, Lei Lamei et al. Effects of temperature, irradiance and nitrate on the growth of Cylindrospermopsis raciborskii N8. J Lake Sci, 2014, 26(3): 441-446. [于婷, 戴景峻, 雷腊梅等. 温度, 光照强度及硝酸盐对拟柱孢藻(Cylindrospermopsis raciborskii N8)生长的影响. 湖泊科学, 2014, 26(3): 441-446. DOI:10.18307/2014.0315] |
| [108] |
Bonilla S, Aubriot L, Soares MCS et al. What drives the distribution of the bloom-forming cyanobacteria P lanktothrix agardhii, and Cylindrospermopsis raciborskii?. Fems Microbiology Ecology, 2012, 79(3): 594-607. DOI:10.1111/fem.2012.79.issue-3 |
| [109] |
Briand JF, Leboulanger C, Humbert JF et al. Cylindrospermopsis raciborskii (Cyanobacteria) invasion at mid-latitudes: Selection, wide physiological tolerance, or global warming?. Journal of Phycology, 2004, 40(2): 231-238. DOI:10.1111/j.1529-8817.2004.03118.x |
| [110] |
Yu Ting. Effects of temperature, light and nitrogen on the growth andtrichome morphology of Cylindrospermopsis raciborskii[Dissertation]. Guangzhou:Jinan University, 2014. [于婷. 温度、光照及氮源对拟柱孢藻生长和藻丝形态的影响[学位论文]. 广州: 暨南大学, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10559-1015002624.htm ]
|
| [111] |
Carneiro RL, Dos Santos MEV, Pacheco ABF. Effects of light intensity and light quality on growth and circadian rhythm of saxitoxins production in Cylindrospermopsis raciborskii(Cyanobacteria). Journal of Plankton Research, 2009, 31(5): 481-488. DOI:10.1093/plankt/fbp006 |
| [112] |
Moisander PH, Lou AC, Braddy J et al. Facultative diazotrophy increases Cylindrospermopsis raciborskii, competitiveness under fluctuating nitrogen availability. FEMS Microbiology Ecology, 2012, 79(3): 800-811. DOI:10.1111/fem.2012.79.issue-3 |
| [113] |
Calandrino ES, Paerl HW. Determining the potential for the proliferation of the harmful cyanobacterium Cylindrospermopsis raciborskii in Currituck Sound, North Carolina. Harmful Algae, 2011, 11: 1-9. DOI:10.1016/j.hal.2011.04.003 |
| [114] |
Moisander PH, Mcclinton E, Paerl HW. Salinity effects on growth, photosynthetic parameters, and nitrogenase activity in estuarine planktonic cyanobacteria. Microbial Ecology, 2002, 43(4): 432-442. DOI:10.1007/s00248-001-1044-2 |
| [115] |
Holland DP, Pantorno A, Orr PT et al. The impacts of a high CO2, environment on a bicarbonate user: The cyanobacterium Cylindrospermopsis raciborskii. Water Research, 2012, 46(5): 1430-1437. DOI:10.1016/j.watres.2011.11.015 |
| [116] |
Shapiro J. Blue-green algae: Why they become dominant. Science, 1973, 179(4071): 382-384. DOI:10.1126/science.179.4071.382 |
| [117] |
Molica RJ, Oliveira EJ, Carvalho PV et al. Occurrence of saxitoxins and an anatoxin-a (s)-like anticholinesterase in a Brazilian drinking water supply. Harmful Algae, 2005, 4(4): 743-753. DOI:10.1016/j.hal.2004.11.001 |
| [118] |
Soto-Liebe K, Méndez MA, Fuenzalida L et al. PSP toxin release from the cyanobacterium Raphidiopsis brookii, D9 (Nostocales) can be induced by sodium and potassium ions. Toxicon Official Journal of the International Society on Toxinology, 2012, 60(7): 1324-1334. DOI:10.1016/j.toxicon.2012.09.001 |
| [119] |
Fastner J, Heinze R, Humpage AR et al. Cylindrospermopsin occurrence in two German lakes and preliminary assessment of toxicity and toxin production of Cylindrospermopsis raciborskii, (Cyanobacteria) isolates. Toxicon, 2003, 42(3): 313-321. DOI:10.1016/S0041-0101(03)00150-8 |
| [120] |
Humpage AR, Fontaine F, Froscio S et al. Cylindrospermopsin genotoxicity and cytotoxicity: Role of cytochrome P-450 and oxidative stress. Journal of Toxicology & Environmental Health Part A, 2005, 68(9): 739-753. |
| [121] |
Moreira C, Azevedo J, Antunes A et al. Cylindrospermopsin: Occurrence, methods of detection and toxicology. Journal of Applied Microbiology, 2013, 114(3): 605-620. DOI:10.1111/jam.2013.114.issue-3 |
| [122] |
Klitzke S, Beusch C, Fastner J. Sorption of the cyanobacterial toxins cylindrospermopsin and anatoxin-a to sediments. Water Research, 2011, 45(3): 1338-1346. DOI:10.1016/j.watres.2010.10.019 |
| [123] |
Chiswell RK, Shaw GR, Eaglesham G et al. Stability of cylindrospermopsin, the toxin from the cyanobacterium, Cylindrospermopsis raciborskii: Effect of pH, temperature, and sunlight on decomposition. Environmental Toxicology, 1999, 14(1): 155-161. DOI:10.1002/(ISSN)1522-7278 |
| [124] |
Stewart I, Seawright AA, Schluter PJ et al. Primary irritant and delayed-contact hypersensitivity reactions to the freshwater cyanobacterium Cylindrospermopsis raciborskii, and its associated toxin cylindrospermopsin. BMC Dermatology, 2005, 6(1): 1-12. |
| [125] |
Banker R, Carmeli S, Werman M et al. Uracil moiety is required for toxicity of the cyanobacterial hepatotoxin cylindrospermopsin. Journal of Toxicology & Environmental Health Part A, 2001, 62(62): 281-288. |
| [126] |
Sukenik A, Hadas O, Kaplan A et al. Invasion of Nostocales (Cyanobacteria) to subtropical and temperate freshwater lakes—Physiological, regional, and global driving forces. Frontiers in Microbiology, 2012(3): 86. |
| [127] |
Sinha R, Pearson LA, Davis TW et al. Comparative genomics of Cylindrospermopsis raciborskii strains with differential toxicities. BMC Genomics, 2014, 15(1): 83. DOI:10.1186/1471-2164-15-83 |
| [128] |
Norris RL, Eaglesham GK, Pierens G et al. Deoxycylindrospermopsin, an analog of cylindrospermopsin from Cylindrospermopsis raciborskii. Environmental Toxicology, 1999, 14(1): 163-165. DOI:10.1002/(ISSN)1522-7278 |
| [129] |
Orr PT, Rasmussen JP, Burford MA et al. Evaluation of quantitative real-time PCR to characterise spatial and temporal variations in cyanobacteria, Cylindrospermopsis raciborskii (Wołoszyńska) Seenaya et Subba Raju and cylindrospermopsin concentrations in three subtropical Australian reservoirs. Harmful Algae, 2010, 9(3): 243-254. DOI:10.1016/j.hal.2009.11.001 |
| [130] |
Wimmer KM, Strangman WK, Wright JL. 7-Deoxy-desulfo-cylindrospermopsin and 7-deoxy-desulfo-12-acetyl cylindrospermopsin: Two new cylindrospermopsin analogs isolated from a Thai strain of Cylindrospermopsis raciborskii. Harmful Algae, 2014, 37(4): 203-206. |
| [131] |
Davis TW, Orr PT, Boyer GL et al. Investigating the production and release of cylindrospermopsin and deoxy-cylindrospermopsin by Cylindrospermopsis raciborskii over a natural growth cycle. Harmful Algae, 2014, 31(31): 18-25. |
| [132] |
Willis A, Chuang AW, Woodhouse JN et al. Intraspecific variation in growth, morphology and toxin quotas for the cyanobacterium, Cylindrospermopsis raciborskii. Toxicon, 2016, 119: 307-310. DOI:10.1016/j.toxicon.2016.07.005 |
| [133] |
Merel S, Walker D, Chicana R et al. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environment International, 2013, 59: 303-327. DOI:10.1016/j.envint.2013.06.013 |
| [134] |
Rzymski P, Poniedziałek B. In search of environmental role of cylindrospermopsin: A review on global distribution and ecology of its producers. Water Research, 2014, 66: 320-337. DOI:10.1016/j.watres.2014.08.029 |
| [135] |
Jones GJ, Negri AP. Persistence and degradation of cyanobacterial paralytic shellfish poisons (PSPs) in freshwaters. Water Research, 1997, 31(3): 525-533. DOI:10.1016/S0043-1354(96)00134-0 |
| [136] |
Christoffersen K, Kaas H. Toxic cyanobacteria in water. A guide to their public health consequences, monitoring, and management. American Society of Limnology & Oceanography, 2000, 45: 1212. |
| [137] |
Nicholson BC, Shaw GR, Morrall J et al. Chlorination for degrading saxitoxins (paralytic shellfish poisons) in water. Environmental Technology, 2003, 24(11): 1341-1348. DOI:10.1080/09593330309385678 |
| [138] |
Su Z, Sheets M, Ishida H et al. Saxitoxin blocks L-type I Ca. Journal of Pharmacology & Experimental Therapeutics, 2004, 308(1): 324-329. |
| [139] |
Landsberg JH. The effects of harmful algal blooms on aquatic organisms. Reviews in Fisheries Science, 2002, 10(2): 113-390. DOI:10.1080/20026491051695 |
| [140] |
Westrick JA, Szlag DC, Southwell BJ et al. A review of cyanobacteria and cyanotoxins removal/inactivation in drinking water treatment. Analytical and Bioanalytical Chemistry, 2010, 397(5): 1705-1714. DOI:10.1007/s00216-010-3709-5 |
| [141] |
Carneiro RL, Pacheco ABF, De Oliveira E et al. Growth and saxitoxin production by Cylindrospermopsis raciborskii (Cyanobacteria) correlate with water hardness. Marine Drugs, 2013, 11(8): 2949-2963. DOI:10.3390/md11082949 |
| [142] |
Merel S, Clément M, Mourot A et al. Characterization of cylindrospermopsin chlorination. Science of the Total Environment, 2010, 408(16): 3433-3442. DOI:10.1016/j.scitotenv.2010.04.033 |
| [143] |
Harada KI, Ohtani I, Iwamoto K et al. Isolation of cylindrospermopsin from a Cyanobacterium Umezakia Natans and its screening method. Toxicon, 1994, 32(1): 73-84. DOI:10.1016/0041-0101(94)90023-X |
| [144] |
Welker M, Bickel H, Fastner J. HPLC-PDA detection of cylindrospermopsin opportunities and limits. Water Research, 2002, 36(18): 4659-4663. DOI:10.1016/S0043-1354(02)00194-X |
| [145] |
Metcalf JS, Beattie KA, Saker ML et al. Effects of organic solvents on the high performance liquid chromatographic analysis of the cyanobacterial toxin cylindrospermopsin and its recovery from environmental eutrophic waters by solid phase extraction. FEMS Microbiology Letters, 2002, 216(2): 159-164. DOI:10.1111/fml.2002.216.issue-2 |
| [146] |
Meriluoto J, Codd GA. Cyanobacterial monitoring and cyanotoxin analysis. Acta Academiae Aboensis, 2005, 65(1): 1-145. |
| [147] |
Wörmer L, Carrasco D, Cirés S et al. Advances in solid phase extraction of the cyanobacterial toxin cylindrospermopsin. Limnology and Oceanography: Methods, 2009, 7(7): 568-575. DOI:10.4319/lom.2009.7.568 |
| [148] |
Eaglesham GK, Norris RL, Shaw GR et al. Use of HPLC-MS/MS to monitor cylindrospermopsin, a blue-green algal toxin, for public health purposes. Environmental Toxicology, 1999, 14(14): 151-154. |
| [149] |
Stirling DJ, Quilliam MA. First report of the cyanobacterial toxin cylindrospermopsin in New Zealand. Toxicon, 2001, 39(8): 1219-1222. DOI:10.1016/S0041-0101(00)00266-X |
| [150] |
Guzmán-Guillén R, Prieto AI, Gonzalez AG et al. Cylindrospermopsin determination in water by LC-MS/MS: Optimization and validation of the method and application to real samples. Environmental Toxicology and Chemistry, 2012, 31(10): 2233-2238. DOI:10.1002/etc.1954 |
| [151] |
Bogialli S, Bruno M, Curini R et al. Monitoring algal toxins in lake water by liquid chromatography tandem mass spectrometry. Environmental Science & Technology, 2006, 40(9): 2917-2923. |
| [152] |
Kikuchi S, Kubo T, Kaya K. Cylindrospermopsin determination using 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES) as the internal standard. Analytica Chimica Acta, 2007, 583(1): 124-127. DOI:10.1016/j.aca.2006.10.007 |
| [153] |
Graham JL, Loftin KA, Meyer MT et al. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern United States. Environmental Science & Technology, 2010, 44(19): 7361-7368. |
| [154] |
Li Zhixuan, Zhao Qianning, Zhang Changli. Determination of cylindrospermopsin in the water column by ultra high performance liquid chromatography-mass spectrometry. Analytical Laboratory, 2012(11): 90-93. [黎志轩, 赵倩宁, 张长立. 超高效液相色谱-串联质谱测定水中的柱孢藻毒素. 分析试验室, 2012(11): 90-93.] |
| [155] |
Bláhová L, Oravec M, Maršálek B et al. The first occurrence of the cyanobacterial alkaloid toxin cylindrospermopsin in the Czech Republic as determined by immunochemical and LC/MS methods. Toxicon, 2009, 53(5): 519-524. DOI:10.1016/j.toxicon.2009.01.014 |
| [156] |
Zhu Luyao. Study on the release and degradation of cylindrospermopsin in killing Cylindrospermopsis raciborskiiby ClO2[Dissertation]. Guangzhou:Guangdong University of Technology, 2014. [朱璐瑶. 二氧化氯杀灭拟柱孢藻过程中藻毒素的释放与降解研究[学位论文]. 广州: 广东工业大学, 2014. http://cdmd.cnki.com.cn/Article/CDMD-11845-1014294271.htm ]
|
| [157] |
Ho L, Dreyfus J, Boyer J et al. Fate of cyanobacteria and their metabolites during water treatment sludge management processes. Science of the Total Environment, 2012, 424(4): 232-238. |
| [158] |
Campbell K, Huet AC, Charlier C et al. Comparison of ELISA and SPR biosensor technology for the detection of paralytic shellfish poisoning toxins. Journal of Chromatography B, 2009, 877(32): 4079-4089. DOI:10.1016/j.jchromb.2009.10.023 |
| [159] |
Mohamed ZA, Alamri SA. Biodegradation of cylindrospermopsin toxin by microcystin-degrading bacteria isolated from cyanobacterial blooms. Toxicon, 2012, 60(8): 1390-1395. DOI:10.1016/j.toxicon.2012.10.004 |
| [160] |
Zamyadi A, Ho L, Newcombe G et al. Fate of toxic cyanobacterial cells and disinfection by-products formation after chlorination. Water Research, 2012, 46(5): 1524-1535. DOI:10.1016/j.watres.2011.06.029 |
| [161] |
Terao K, Ohmori S, Igarashi K et al. Electron microscopic studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue-green alga Umezakia natans. Toxicon, 1994, 32(7): 833-843. DOI:10.1016/0041-0101(94)90008-6 |
| [162] |
Hiripi L, Nagy L, Kalmár T et al. Insect (Locusta migratoria migratorioides) test monitoring the toxicity of cyanobacteria. Neurotoxicology, 1998, 19(4/5): 605-608. |
| [163] |
Kiss T, Vehovszky Á, Hiripi L et al. Membrane effects of toxins isolated from a cyanobacterium, Cylindrospermopsis raciborskii, on identified molluscan neurones. Comparative Biochemistry & Physiology Part C Toxicology & Pharmacology, 2002, 131(2): 167-176. |
| [164] |
Metcalf JS, Lindsay J, Beattie KA et al. Toxicity of cylindrospermopsin to the brine shrimp Artemia salina: Comparisons with protein synthesis inhibitors and microcystins. Toxicon, 2002, 40(8): 1115-1120. DOI:10.1016/S0041-0101(02)00105-8 |
| [165] |
Nogueira IC, Saker MS, Wiegand C et al. Toxicity of the cyanobacterium Cylindrospermopsis raciborskii to Daphnia magna. Environmental Toxicology, 2004, 19(5): 453-459. DOI:10.1002/(ISSN)1522-7278 |
| [166] |
Gutiérrez-Praena D, Ángeles Jos, Pichardo S et al. Time-dependent histopathological changes induced in Tilapia (Oreochromis niloticus) after acute exposure to pure cylindrospermopsin by oral and intraperitoneal route. Ecotoxicology & Environmental Safety, 2012, 76(2): 102-113. |
| [167] |
Vasas G, Gáspár A, Surányi G et al. Capillary electrophoretic assay and purification of cylindrospermopsin, a cyanobacterial toxin from Aphanizomenon ovalisporum, by plant test (Blue-Green Sinapis Test). Analytical Biochemistry, 2002, 302(1): 95-103. DOI:10.1006/abio.2001.5525 |
| [168] |
Runnegar MT, Kong SM, Zhong YZ et al. The role of glutathione in the toxicity of a novel cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochemical and Biophysical Research Communications, 1994, 201(1): 235-241. DOI:10.1006/bbrc.1994.1694 |
| [169] |
Neumann C, Bain P, Shaw G. Studies of the comparative in vitro toxicology of the cyanobacterial metabolite deoxycylindrospermopsin. Journal of Toxicology & Environmental Health Part A, 2007, 70(19): 1679-1686. |
| [170] |
Froscio SM, Humpage AR, Burcham PC et al. Cell-free protein synthesis inhibition assay for the cyanobacterial toxin cylindrospermopsin. Environmental Toxicology, 2001, 16(5): 408-412. DOI:10.1002/(ISSN)1522-7278 |
| [171] |
Froscio SM, Humpage AR, Wickramasinghe W et al. Interaction of the cyanobacterial toxin cylindrospermopsin with the eukaryotic protein synthesis system. Toxicon, 2008, 51(2): 191-198. DOI:10.1016/j.toxicon.2007.09.001 |
| [172] |
Li Shaoxiu, Xia Wenqin, Zhao Dejun et al. Killing Cylindrospermopsis with chlorine dioxide. Environmental Science & Technology, 2012, 35(6): 152-156. [李绍秀, 夏文琴, 赵德骏等. 二氧化氯杀灭拟柱孢藻的研究. 环境科学与技术, 2012, 35(6): 152-156.] |
| [173] |
Li Shaoxiu, Zhao Dejun, Wang Zhihong et al. Study on the generation of the organic by-products from killing algae with chlorine dioxide. Journal of Safety and Environment, 2016(2): 258-261. [李绍秀, 赵德骏, 王志红等. 二氧化氯杀灭拟柱孢藻生成有机副产物的研究. 安全与环境学报, 2016(2): 258-261.] |
| [174] |
Maghsoudi E, Prévost M, Duy SV et al. Adsorption characteristics of multiple microcystins and cylindrospermopsin on sediment: Implications for toxin monitoring and drinking water treatment. Toxicon, 2015, 103: 48-54. DOI:10.1016/j.toxicon.2015.06.007 |
| [175] |
Klitzke S, Fastner J. Cylindrospermopsin degradation in sediments—The role of temperature, redox conditions, and dissolved organic carbon. Water Research, 2012, 46(5): 1549-1555. DOI:10.1016/j.watres.2011.12.014 |
| [176] |
Mouchet P, Bonnelye V. Solving algae problems: French expertise and world-wide applications. Journal of Water Supply: Research and Technology-AQUA, 1998, 47(3): 125-141. DOI:10.1046/j.1365-2087.1998.00091.x |
| [177] |
Dixon MB, Falconet C, Ho L et al. Nanofiltration for the removal of algal metabolites and the effects of fouling. Water Science & Technology, 2010, 61(5): 1189-1199. |
| [178] |
Dixon MB, Falconet C, Ho L et al. Removal of cyanobacterial metabolites by nanofiltration from two treated waters. Journal of Hazardous Materials, 2011, 188(1): 288-295. |
| [179] |
Rodríguez E, Onstad GD, Kull TPJ et al. Oxidative elimination of cyanotoxins: Comparison of ozone, chlorine, chlorine dioxide and permanganate. Water Research, 2007, 41(15): 3381-3393. DOI:10.1016/j.watres.2007.03.033 |
| [180] |
Rodríguez E, Sordo A, Metcalf JS et al. Kinetics of the oxidation of cylindrospermopsin and anatoxin-a with chlorine, monochloramine and permanganate. Water Research, 2007, 41(9): 2048-2056. DOI:10.1016/j.watres.2007.01.033 |
| [181] |
Rodríguez E, Majado ME, Meriluoto J et al. Oxidation of microcystins by permanganate: Reaction kinetics and implications for water treatment. Water Research, 2007, 41(1): 102-110. DOI:10.1016/j.watres.2006.10.004 |
| [182] |
Tratnyek PG, Johnson RL. Nanotechnologies for environmental cleanup. Nano Today, 2006, 1(2): 44-48. DOI:10.1016/S1748-0132(06)70048-2 |
| [183] |
Manual EG. Alternative disinfectants and oxidants guidance manual. US EPA, 1999.
|
| [184] |
Ho L, Slyman N, Kaeding U et al. Optimizing PAC and chlorination practices for cylindrospermopsin removal. American Water Works Association Journal, 2008, 100(11): 88. |
| [185] |
Ho L, Lambling P, Bustamante H et al. Application of powdered activated carbon for the adsorption of cylindrospermopsin and microcystin toxins from drinking water supplies. Water Research, 2011, 45(9): 2954-2964. DOI:10.1016/j.watres.2011.03.014 |
| [186] |
Senogles P, Smith MJ. Physical, chemical and biological methods for the degradation of the cyanobacterial toxin, cylindrospermopsin. American Water Works Association, 2002.
|
| [187] |
Smith MJ, Shaw GR, Eaglesham GK et al. Elucidating the factors influencing the biodegradation of cylindrospermopsin in drinking water sources. Environmental Toxicology, 2008, 23(3): 413-421. DOI:10.1002/(ISSN)1522-7278 |
| [188] |
Klitzke S, Apelt S, Weiler C et al. Retention and degradation of the cyanobacterial toxin cylindrospermopsin in sediments—The role of sediment preconditioning and DOM composition. Toxicon Official Journal of the International Society on Toxinology, 2010, 55(5): 999-1007. DOI:10.1016/j.toxicon.2009.06.036 |
| [189] |
Maghsoudi E, Fortin N, Greer C et al. Biodegradation of multiple microcystins and cylindrospermopsin in clarifier sludge and a drinking water source: Effects of particulate attached bacteria and phycocyanin. Ecotoxicology & Environmental Safety, 2015, 120: 409-417. |
| [190] |
Dziga D, Kokocinski M, Maksylewicz A et al. Cylindrospermopsin biodegradation abilities of Aeromonas sp. isolated from Rusalka Lake. Toxins, 2016, 8(3): 55. DOI:10.3390/toxins8030055 |
| [191] |
He Yazi, Pan Weibin, He Jiahui et al. Effect of algae lysing bacteria L7 on the algal community in eutrophic water. Environmental Protection Science, 2013, 39(6): 25-29. [何雅孜, 潘伟斌, 何嘉辉等. 溶藻细菌L7对富营养化水体藻类群落的影响. 环境保护科学, 2013, 39(6): 25-29.] |
| [192] |
Song W, Yan S, Cooper WJ et al. Hydroxyl radical oxidation of Cylindrospermopsin (Cyanobacterial toxin) and its role in the photochemical transformation. Environmental Science & Technology, 2012, 46(22): 12608-12615. |
| [193] |
Senogles PJ, Scott JA, Shaw G et al. Photocatalytic degradation of the cyanotoxin cylindrospermopsin, using Titanium Dioxide and UV Irradiation. Water Research, 2001, 35(5): 1245-1255. DOI:10.1016/S0043-1354(00)00372-9 |
| [194] |
Chen L, Zhao C, Dionysiou DD et al. TiO2 photocatalytic degradation and detoxification of cylindrospermopsin. Journal of Photochemistry and Photobiology A: Chemistry, 2015, 307: 115-122. |
| [195] |
He X, Zhang G, de la Cruz AA et al. Degradation mechanism of cyanobacterial toxin cylindrospermopsin by hydroxyl radicals in homogeneous UV/H2O2 process. Environmental Science & Technology, 2014, 48(8): 4495-4504. |
| [196] |
Senogles PJ, Scott JA, Shaw G. Efficiency of UV treatment with and without the photocatalyst titanium dioxide for the degradation of the cyanotoxin cylindrospermopsin. Resource and Environmental Biotechnology, 2000, 3(2/3): 111-125. |
| [197] |
Bandala ER, MartíNez D, MartíNez E et al. Degradation of microcystin-LR toxin by Fenton and Photo-Fenton processes. Toxicon Official Journal of the International Society on Toxinology, 2004, 43(7): 829-832. DOI:10.1016/j.toxicon.2004.03.013 |
| [198] |
Yan S, Jia A, Merel S et al. Ozonation of Cylindrospermopsin (Cyanotoxin): Degradation mechanisms and cytotoxicity assessments. Environmental Science & Technology, 2016, 50(3): 1437-1446. |
| [199] |
Momani FA, Smith DW, El-Din MG. Degradation of cyanobacteria toxin by advanced oxidation processes. Journal of Hazardous Materials, 2008, 150(2): 238-249. DOI:10.1016/j.jhazmat.2007.04.087 |
| [200] |
Momani FA. Degradation of cyanobacteria anatoxin-a by advanced oxidation processes. Separation & Purification Technology, 2007, 57(1): 85-93. |
| [201] |
Hoeger SJ, Shaw G, Hitzfeld BC et al. Occurrence and elimination of cyanobacterial toxins in two Australian drinking water treatment plants. Toxicon, 2004, 43(6): 639-649. DOI:10.1016/j.toxicon.2004.02.019 |
| [202] |
Yang X, Lin J, Sun T et al. Mechanisms and factors influencing the removal of microcystin-LR by ultrafiltration membranes. Journal of Membrane Science, 2008, 320(1/2): 240-247. |
| [203] |
Ianelli R, Bianchi V, Carducci A et al. Effects of intracellular/dissolved ratios of microcystin-LR on its removal by ultrafi ltration. Desalination and Water Treatment, 2010, 23(1/2/3): 152-160. |
 2017, Vol. 29
2017, Vol. 29 


