(2: 中国科学院水生生物研究所东湖湖泊生态系统试验站, 武汉 430072)
(3: 江西省环境保护科学研究院, 南昌 330029)
(2: Donghu Experimental Station of Lake Ecosystem, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, P. R. China)
(3: Jiangxi Academy of Environmental Sciences, Nanchang 330029, P. R. China)
近半世纪以来,国内外关于水体富营养化所诱发的水生植物衰退的学术活动与报道层出不穷,探讨富营养化水体中水生植物衰退已然成为当今水生态学研究的一个热点.富营养化水体中水生植物衰退主要原因是来自于富营养化水体中过量氮磷等营养元素和由这些营养增加所引发浮游植物的大量生长繁殖以及这些浮游植物过量生长所导致的水体可利用光和溶解氧的减少、藻毒素、硫化物等级联效应方面,对水生植物的形态、生物量分配、组织结构和代谢等方面产生显著影响[1-6],从而抑制了水生植物的生长,限制了最大分布水深,阻断了其生活史,最终导致富营养化水体中水生植物的大面积衰退[7-9].
生物力学性能是指运用力学原理和方法研究生命系统的结构与功能的一类指标,它是衡量流体环境中生命系统与环境因子(如重力、风力、土壤、水流、波浪等)和生物因子(如植物、动物和微生物等)的相互作用过程(如接触、挤压、粘附、渗透、捕捉或传输等物理作用)中的行为特征、适应能力和作用程度的重要变量[10-20].尽管水生植物应对水体富营养化的响应研究主要集中在生长、形态、生物量分配、组织结构和代谢等方面[1-6],近年来一系列研究表明水体富营养化带来的底泥富营养、水体高浓度氮磷和可利用光缺乏等主要环境变量均可显著影响水生植物的生物力学性能[21-25].水生植物生物力学性能是水生植物适应水生生境过程中无时无处不在的,由波浪、流速和食草动物等引起的机械胁迫的主要指标[26-28].本文以水生植物的生物力学性能为核心,综述了水生植物的生物力学性能特征及其对水体富营养化的响应,并总结分析其在富营养化水体中对水生植物衰退的贡献等.
1 水生植物的生物力学性能特征 1.1 水生境的机械胁迫类型及其特征不同于陆生植物所受的机械胁迫类型,水生植物尤其是根生水生植物所受机械胁迫类型主要有波浪、水流、船舶以及食草动物等[29-30].但由于水体相对的高密度(淡水和海水密度分别是空气密度的833和854倍[31]),同一物体所受到2 m/s水流速度的作用力相当于58 m/s风速的作用力,即:相同速度下,水生境的机械胁迫作用力约是陆生生境机械胁迫作用力的29倍[26, 32-33].
由风带来的波浪、水流等类型的机械胁迫大小与湖泊、河流、海岸带等水体的水深、受风面湖泊的长度和湖盆坡度等[26, 29, 34-36]有关;而波浪、流速等机械胁迫作用于水生植物植株上的作用力大小还与植物密度、植株种类(如生长型[37-38]和叶形[39-40])、植株大小[41-42]以及植株立地角度(即植株底部半米与底泥表面所成的角度,立地角度从40°到70°时所受的机械胁迫力是垂直时的1/2到2/3[29])密切相关.
1.2 水生植物所受的机械胁迫力及其对水生植物的影响水生生境中波浪、水流等机械胁迫主要产生3种作用于植株上的机械胁迫作用力(图 1):平行于水流的拖曳作用力(FD, drag force)、速度力(FA, accelerational force)以及垂直于水流的抬升作用力(FL, lift force)[36, 41-43],其中FD为主要作用力,而FA在水流速度增加或降低时,其方向也与FD方向一致或相反[44].由于水体浮力大,其他大多根生水生植物,如:沉水植物、浮叶植物,通常不需要强壮的茎来支撑植株本身重量,而且具有很大的柔韧性,使得植株能够沿着机械胁迫方向弯曲并通过重构改变植株的大小和形态[45],以减少机械胁迫与植株表面的摩擦力与接触面积,最终降低作用于植株上的机械胁迫力[41-42].因而,一般认为在水流运动方向不固定的水环境中,大多根生水生植物受到的主要机械胁迫作用力是沿植株方向的拖曳力,起到拉伸植株的作用,即造成拉伸胁迫(σ+)[46-48],故又称拉伸作用力[45].而挺水植物,类似大多数陆生植物(作物、树木等)需要强壮的茎来支撑植株重量,即重力(FG,gravitational force),以便使植株保持直立,因而尽管植株沉没在水中和暴露在空气中的部分所受机械胁迫分别来自波浪和风,但其作用于植株上的作用力仍为平行于波浪、水流或风的机械作用力,即拖曳作用力,但表现为垂直于植株方向的弯曲作用力(类似于作用于其他水生植物上的平行于水流的拖曳作用力)[49-50],即由受风面的拉伸(σ+)和背风面的挤压(σ-)组合而成[46, 48](图 1).此外,值得一提的是外来种粉绿狐尾藻(Myriophyllum aquaticum (Vell.) Verdcourt),它主要生长在沼泽区或经常水位波动的静水或缓流水体的浅水区,但能够通过异形叶性(气生叶和沉水叶)来适应水陆生境转化,最长能够耐受9个月的只有土壤基质保持水饱和状态低水位时期[51-53],因此我们推测它所受的主要机械胁迫作用力仍是平行于风、波浪、水流的作用力,但当植株完全淹没水中时该作用力表现为平行于植株的拖曳作用力(类似于大部分水生植物),但当植株部分/全部暴露于空气中时表现为垂直于植株的弯曲作用力(类似于挺水植物).事实上随着全球气候变化引发的异常降雨情势和水利工程调控的水文节律使得水生态系统,如湖泊、河流、湿地、海岸带等洪水期淹水和枯水期退水的水深变化节律发生明显变化[48, 54-56],使得水生植物的生境发生水陆生境转换,其中沉水植物的生境会在长期干枯之后面临着陆地生境的机械胁迫,而挺水植物则会在长期的洪水期面临着完全水生境拖曳力,这些因水陆生境导致机械胁迫作用到植株上的机械胁迫作用力,即拖曳力弯曲作用力间转换,会挑战植物长期适应各自生境形成的生理生态和生物力学适应性,进而打破现有的水生植物群落结构(图 1)[48].
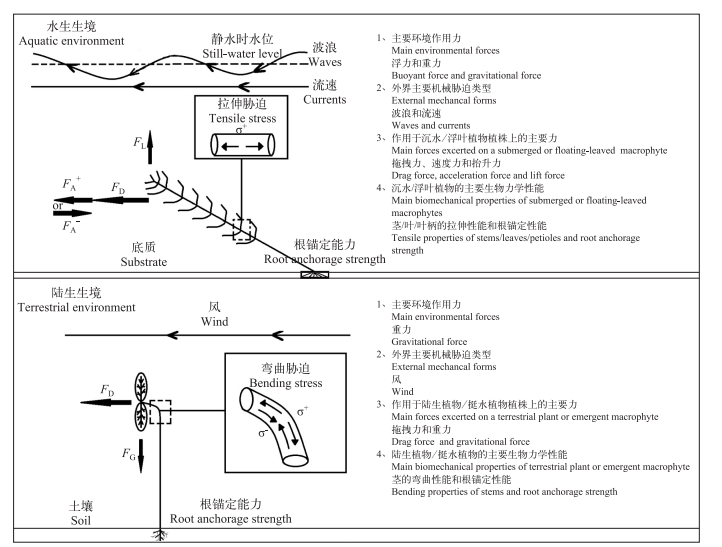
|
图 1 水生生境和陆生生境中各自的主要环境作用力、机械胁迫类型(细箭头表示)、作用于植株上的主要力(粗箭头表示)及植株的主要生物力学性能示意图(改自文献[44, 46-48]) Fig.1 Schematic overview of the main environmental forces, mechanical forms (thin arrows) and main forces (thick arrows) acting on a sessile plant in aquatic and terrestrial environment as well as the main biomechanical properties of plants |
尽管大多水生植物能够随水流摆动避开垂直水流的抬升力,一旦波浪、水流等机械胁迫作用于植株上的拖曳力超过水生植物本身的柔韧性能临界值,植株就会发生断枝、拔根等机械损伤[26, 39, 43].其中,断枝,尤其是植株具有顶芽或顶端分生组织的顶段部位的断裂,会造成植株生物量、能量和光合作用能力多重损失[23, 57-59];且这种由机械胁迫造成的非主动断枝的扩散和定植能力远低于该种植物在生长阶段后期(当植株生物量达最大值后完成须根、细胞木质化和碳水化合物等准备时)自发形成的主动断枝的扩散和定植能力[53, 60-61],因为机械胁迫造成的断枝损失最终会影响水生植物的生存、繁殖、分布和群落结构[31, 37].拔根一般有根断裂、须根从底泥中滑脱出来、根系整体被移除3种情况[26, 62],对水生植物而言主要是根滑脱或根整个系统被移除方式的拔根[26].拔根不仅会严重损伤水生植物尤其是多年生水生植物依靠地下组织进行繁殖的能力,直接导致挺水植物和浮叶植物的死亡,还会增加水生植物恢复工程的定植难度[23, 37, 63].
1.3 水生植物的主要生物力学性能特征水陆生境的差异造成水陆植物生长、形态和组织结构等方面的巨大差异:大多数陆生植物(作物、树木等)和挺水植物主要靠植物的茎支撑植株重量,使植株保持直立;生活史上基本完全淹水的根生水生植物(如浮叶植物和沉水植物)由于水体的浮力作用,类似于攀爬、匍匐陆生植物,不需要茎/叶/叶柄的支撑就能使植株在静水时保持直立状态,但需要茎/叶/叶柄有足够的柔韧性使得植株能够承受水体流动带来的一定程度的拖曳负荷[41-42, 49-50].因而大多数陆生和挺水植物采取以提高抗弯性能为代表的“强度和硬度”机械抵抗应对策略,而完全淹水的根生水生植物则类似陆生攀爬匍匐植物采取以提高抗拉性能为代表的“柔韧和延展”机械抵抗应对策略来适应各自生境的机械胁迫类型(图 1)[28, 64].然而,由于全球气候变化和人类活动日益加剧的双重作用会导致湖滨带、河岸、湿地、海岸带等水陆生境转变节律发生变化[48, 54-56],沿岸带水生植物需要提升以抗弯性能为代表的“强度和硬度”(机械抵抗应对策略使植株能直立生长以适应枯水期短暂出现的陆生生境条件下的机械胁迫)[48].
与陆生植物类似,根生水生植物也需要通过根的锚定作用和底泥黏着作用来固定植株[26, 37, 65],这在强波浪、暴雨以及植被生态修复初期至关重要,没有足够的定植能力,植株往往会被大面积连根拔起,最终影响生长、分布、繁殖、甚至存活.陆生植物绝大多数养分仅来源于土壤基质,但根生水生植物不仅可通过根系从底泥沉积相中吸取营养,还能通过茎叶等营养组织器官从上覆水相中获取营养[2, 66].加上水体浮力很大,除挺水植物存在类似作物、树木等陆生植物保持植株直立状态的主根外,其他水生植物的根与攀爬和匍匐植物的根类似, 均为细且密集的须根[26, 30],这对大部分水生植物而言不仅可拥有较大的比表面积来增加营养物质吸收,而且还能减少整个植株根生物量分配比率,将利于植株伸长和可利用光获取能力增长.因而,除挺水植物外,水生植物的根类似于攀爬和匍匐植物的根, 主要受到拉伸作用力,且其根锚定强度与整个根系水平分布范围以及底泥的类型有关[26, 67].
2 水生植物生物力学性能对水体富营养化的响应由人类活动(城市污水、农业污水排放等)带来的水体富营养化主要体现在以硝态氮、铵态氮为主要形态的高浓度氮和以正磷酸盐为主要形态的高浓度磷[68-69].水体高浓度营养物质促进藻类过度生长繁殖,从而降低水体可利用光,而长时间的沉积作用会导致底泥营养水平的增加, 进而影响底泥颗粒结构、溶解氧水平、微生物群落组成和黏着能力[26, 70-71].研究表明富营养化带来的底泥营养类型、水体营养水平和水体可利用光多少等主要环境变量均会显著影响水生植物的生物力学性能.
2.1 富营养化底泥对水生植物生物力学性能的影响研究表明,水体富营养化生境的底泥不仅营养水平高,其黏着力(<0.2 kPa)也比其他营养水平水体底泥的(>1.0 kPa)小[26],生长在富营养化底泥的水生植物根系也通常较短较少[2-3, 24, 66],而底泥黏着力和植株根系是决定植株根锚定性能的主要指标(表 1)[26-27, 72],因而生长在富营养化底泥中的植物具有较低的根锚定性能[26],极易发生根滑脱或根整个系统被移除的拔根机械损伤,不利于富营养化水体中水生植物修复和重建工程中植被的定植以及受机械损伤的植株地下组织储藏物质的保存.
| 表 1 与水陆生植物根锚定性能相关的根形态(改自文献[73]) Tab.1 Morphological traits positively correlated with root anchorage strength of plants |
与陆生植物类似[92-94],通常生长在中营养水平底泥的植株较生长在富营养底泥的植株的茎/叶/叶柄具有较高的生物力学性能[22-25].本人前期室内研究发现与富营养底泥(总磷(TP)=1.40 mg/g DW、总氮(TN)= 6.32 mg/g DW、有机质(OM)=11.70 %)和贫营养底泥(0.1 cm细砂:TP、TN和OM含量均低于检测限)相比,中营养水平底泥(TP=0.70 mg/g DW、TN=3.41 mg/g DW、OM =8.13 %)更有利于轮叶黑藻(Hydrilla verticillata)形成更大的抗拉应力和弯曲能力[23];而富营养水平底泥(TP=2.43 mg/g DW、TN=3.56 mg/g DW、OM=7.70 %)较中营养水平底泥(TP=0.57 mg/g DW、TN=2.44 mg/g DW、OM=4.15 %)更有利于穗状狐尾藻(M. spicatum)形成较大的抗拉应力、弯曲应力和较小的拉伸率和结构刚性[24-25];但野外调查发现:随着湖泊营养水平增加,穗状狐尾藻的抗拉应力和拉伸率以及篦齿眼子菜(Potamogeton pectinatus)的抗拉应力均显著增加;而篦齿眼子菜的拉伸率在中营养水平湖泊最大,微齿眼子菜(P. maackianus)的抗拉应力和拉伸率则不受湖泊营养水平的显著影响[25]. La Nafie等[22]野外原位实验发现,与原位基质相比,通过添加0.5 kg/m2缓释肥(N:P:K=18:9:3; Osmocote®)模拟获得的富营养水平底泥会降低喜盐草(Halophila ovalis)的抗拉强度而增加其拉伸率,而对二药藻(Halodule uninervis)的抗拉性能没有显著影响.这一方面可能是由于植株的生物力学性能与其形态特征密切相关,如在上述各研究中各水生植物的茎/叶不仅在生物力学性能方面,也在形态(茎的横截面面积或叶的长、宽和厚度)方面对底泥营养水平具有不同程度的响应[22-24],其中常生长在沙质底泥的海草如喜盐草叶片长度、宽度和厚度均随底泥营养水平增加而增加,而能够在多种类型底泥生长的海草如二药藻的叶子宽度和厚度则没有显著变化[22];另一方面也可能与两种植物本身的生长环境有关,喜盐草本身多生长在砂质基质,而二药藻则能在多种基质上生长[95].
2.2 水体营养水平对水生植物生物力学性能的影响水体营养水平对水生植物的生物力学性能具有显著影响,且具有种间差异[21, 25, 63].例如:在高营养水平水体(硝态氮(NO3--N): 1.94 mg/L;铵态氮(NH4+-N): 21.84 μg/L;正磷酸盐磷(PO43--P): 128.47 μg/L)中生长的挺水植物水薄荷(Mentha aquatica L.)和沼泽勿忘草(Myosotis scorpioides L.)的抗弯性能比生长在低营养水平水体(NO3--N: 1.94 mg/L;NH4+-N: 4.09 μg/L;PO43--P: 24.35 μg/L)植株的分别小约20 %和50 % [21];而生长在中-富营养水平海水(NO3--N: 3.41 mg/L;NH4+-N: 0.15 μg/L;PO43--P: 0.95 μg/L)[68]的诺氏大叶藻(Zostera noltii)较生长在正常海水的植株具有较小的抗拉性能[63].
大量研究表明,高浓度NH4+-N是富营养化水体对水生植物影响最大的营养因素[1, 5, 96-97].祝国荣等[24]研究发现与富营养底泥相比,水体高NH4+-N浓度对穗状狐尾藻茎的抗拉性能和抗弯性能以及根的锚定性能的影响更大,且二者间具有协同作用.这一方面可能是因为水生植物尤其是沉水植物可以根据底泥的营养水平调节自身的根/茎比例,生长在富营养生境的植株通常会降低地下地上比值、地下部分生物量比例、根生物量、须根数和最长须根长[2-3, 24-26, 66],因而植株所受富营养化底泥影响的部位较受水体高浓度NH4+-N影响的部位小很多;另一方面也与富营养化底泥除了高浓度氮还存在高浓度磷有关,研究表明磷可促进植株通过碳水化合物的输出将NH4+-N转化为游离氨基酸来缓解水体高浓度NH4+-N导致的植物组织铵中毒[98-99].
2.3 富营养化水体可利用光缺乏对水生植物生物力学性能的影响植物可利用光的缺乏是富营养化引发藻类过量生长带来的重要结果[8, 100],不仅显著地影响水生植物的形态和生理生化过程[1, 3-5, 99],也会显著地影响水生植物的生物力学性能[22, 59]. La Nafie等[22]研究发现遮光处理显著增加二药藻的拉伸率而降低其抗拉应力;但与之相比,遮光条件下喜盐草具有相对较大的抗拉应力和较小的拉伸率.祝国荣等[59]通过野外原位浮床实验发现在随着水深带来的低可利用光(15.416~290.268 μmol/(m2·s))生境下,5种实验水生植物中,只有金鱼藻(Ceratophyllum demersum)能够在一定程度上调整其生物力学性能,例如增加其茎抗拉性能和根的锚定性能(其根系则在中度水深区最大,此时可利用光约为69.372 μmol/(m2·s))来适应此生境变化;而微齿眼子菜、穗状狐尾藻、轮叶黑藻和竹叶眼子菜(P. malaianus)这4种水生植物的根系和茎的生物力学性能(抗拉应力和/或拉伸率)呈显著降低趋势;此外,轮叶黑藻和竹叶眼子菜的生物力学性能在浅水区时最大,在中度水深区最小,而微齿眼子菜则在中度水深区具有最大的茎生物力学性能.
3 在水体富营养化条件下生物力学性能变化在水生植物衰退中的作用 3.1 生物力学损伤对水生植物的影响根据水动力学研究,水生境机械胁迫力随着水深呈指数下降[37, 43],这表明,处于任何水深的根生水生植物都会受到一定的机械胁迫作用力,而且茎/叶/叶柄的底段部分所受到的机械作用力远远小于其他部位,尤其是顶部.而水生植物的个体发生学的差异会造成水生植物茎/叶/叶柄等部位从底端到顶端的物质含量和解剖结构间存在显著差异[23, 30],因而,水生植物的茎/叶/叶柄的生物力学性能从底端到顶端具有显著差异[23-24, 30].水生植物的茎/叶/叶柄的生物力学性能、根锚定性能与机械胁迫作用力的大小共同决定着植株是否会发生机械损伤、发生何种类型机械损伤(如:断枝、拔根等)以及发生机械损伤的程度(植株的顶段、中段或底段;大型海藻的固着器、柄部或叶片)(图 2)[26, 101-102].
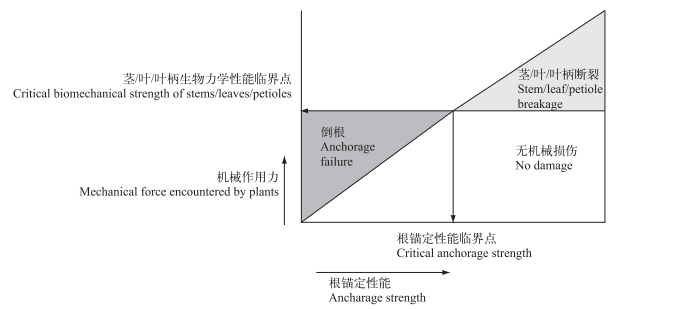
|
图 2 根生水生植物在面临波浪、水流等机械胁迫时,由于作用于植株上的作用力、植株根锚定性能和茎/叶/叶柄的生物力学性能不同,会出现的机械损伤情况(改自文献[26]) Fig.2 Conceptual model showing the potential fates of a rootedmacrophyte subjected to mechanical force encountered by aquatic macrophytes, as cohesive strength of the sediment, root anchorage strength, and the biomechanical indices of stems/leaves/petioles varies |
尽管通过断枝或地下组织如根状茎、匍匐茎、块茎等营养器官进行的营养繁殖是水生植物的重要繁殖扩散方式[26],且前期大多数研究认为茎底段断裂是对根部等地下组织器官的保护[26, 30, 65],不仅能够增强受机械损伤的植株通过地下组织器官再次萌发生长的能力,也通过增加断枝的长度和生物量提高了断枝的扩大和再生能力[103].但是与主动断枝不同,机械胁迫带来的非主动断枝因为缺乏主动断枝形成前期的物质能量储备和须根的形成,通常具有较低的扩散、定植、发芽等再生能力[60-61, 104].因而,在富营养化生境,相对底段断裂而言,更易发生具有顶芽或顶端分生组织和叶密集生长区的顶段断枝.有研究表明[105], 10 % ~30 %和70 %的叶子损伤可分别降低其饱和光合速率至40 %和60 %,这预示着断枝后的植株会有生物量、能量和光合作用能力的多重损失[23, 57-59],不利于断枝后植株的再生;此外,研究还表明植株的大小与主动断枝的生成呈显著正相关[104],而富营养化生境通常形成较小的植株[24, 63, 98],这可能预示着富营养化生境会产生较少的主动断枝和较多的非主动断枝,从而降低水生植物的无性繁殖能力.
富营养化生境由于底泥疏松对植株根系的黏着力非常低[26],与此同时富营养化底泥会显著抑制水生植物根的生长[2-3, 24, 66],形成不发达的根系,最终降低植株的根锚定性能,使得富营养化生境的水生植物更易发生拔根机械损伤[26].拔根对水生植物的影响不仅预示着地下组织的机械损伤和根部生物量的损失,还意味着水生植物尤其是多年生水生植物依靠地下组织进行繁殖能力的丧失,这也可能是水生植物生物力学性能研究最初只关注茎/叶/叶柄底部的拉伸性能或弯曲性能的主要原因[26, 38, 64].而拔根后的水生植物的生存能力因生活型不同而可能不同:对于沉水植物而言,拔根后意味着初始用于来年或者合适条件种群扩展而萌发新植株的地下组织,被迫随水流飘荡直至到达合适生境定植再生,虽然其生存能力因拥有地下组织而比非主动断枝强很多,但这拔根机械损伤毕竟是以原生境的植株为代价,故认为植株具有较小的生物力学性能的茎/叶/叶柄底部是保存原生境植株生存和繁殖能力的“机械引信(mechanical fuse)”[15, 26, 30, 65];而挺水植物和浮叶植物不仅依靠根获取营养,还需要暴露于空气中的部分进行光合作用,而发生拔根机械损伤后的植株既不能通过根获得足够养分生长,也几乎丧失了地上部分的光合作用能力,且漂浮在水体中拔根植株难以直立极易发生腐烂[106],因而拔根对它们而言更多地意味着死亡.此外,拔根还不利于该水域中水生植物恢复工程的定植[23, 37, 63],从而增加水生植物修复工程的难度,不利于富营养水体植被的重建和整个生态系统的恢复.
3.2 生物力学性能与受富营养化显著影响的其他方面的协同作用水生植物的生物力学性能不仅受水体富营养化进程中的富营养化底泥、水体高浓度氮磷和可利用光缺乏3大要素的显著影响[22-24],而且与受富营养化水体显著影响的水生植物形态、生物量分配、组织结构、物质含量等方面密切相关[23-24, 30, 32-33].
3.2.1 受富营养化生境影响的水生植物形态与生物力学间的关联以底泥富营养化、水体高浓度氮磷和可利用光缺乏为主要特征的富营养生境:通常当营养超过一定阈值时,一方面会显著抑制水生植物的生长,形成分枝少、茎叶狭长的矮小植株[1, 5, 24-25, 63, 98],且这些细长茎/叶通常比较脆弱易发生断裂[22, 59];另一方面会显著抑制植株根系的生长(例如较少的根数、较短的根、较小的根表面积和根分布范围)从而增加地上生物量的分配以便获得更多的可利用光、二氧化碳等资源[2-3],而较低的地下/地上生物量比例和较低的根系,则预示着较低的植株根锚定性能[24, 26].
3.2.2 受富营养化生境影响的水生植物的组织结构和物质含量与生物力学间的关联以底泥富营养化、水体高浓度氮磷和水下可利用光缺乏为主要特征的富营养生境会显著降低植株的茎/叶/叶柄组织密度、机械组织比例,增加通气组织[6, 21],从而降低植株茎/叶/叶柄的生物力学性能[6, 14, 21, 50];值得注意的是富营养化生境也会造成水生植物的碳、氮、磷等代谢失调,影响其在根、茎、叶等部位间的分配情况,为了缓解富营养化生境造成的低光、低氧和高铵胁迫,植物会将淀粉、可溶性总糖等非结构性碳水化合物作为物质和能量大量消耗,以降低植株体内过量的铵离子和增加植物纤维素和木质素等结构性物质合成来提高耐受能力[1, 5-6];有研究表明作为植物细胞壁主要成分的纤维素和木质素等结构性碳水化合物和作为能量存储物质的淀粉、可溶性总糖等非结构性碳水化合物的含量通常与植株的生物力学性能呈显著正相关[6, 23-24, 50, 107].
3.3 受富营养化影响的水生植物各方面与植株适合度的关联水生植物的生长、形态、代谢、生物力学等均对植株的生存和繁殖能力,即适合度,具有一定影响,且相关功能间存在一定的权衡.
尽管在水体富营养化进程中,在早期贫营养阶段,一些可以耐受低营养胁迫的植物种类(例如轮藻类Characeae和水韭类Isoetids)最先定居,营养水平的初始增加会促进植株的生长、种群的扩展和物种多样性的增加(例如微齿眼子菜、苦草等)[23],但随着营养水平进一步增加会首先抑制喜好生长在贫、中营养水体的物种,即不耐污种的生长和扩散,从而降低物种多样性,形成以耐污种(金鱼藻、穗状狐尾藻、篦齿眼子菜等)为优势种的群落结构,趋于水生植物多样性较低的生态系统,如果营养水平进一步增加,形成富营养型、超富营养生境,这些耐污种的生存和繁殖也会严重受损[25, 108-109].总体而言,富营养化生境通常会抑制植株生长、降低植株高度、减少分枝数量和降低分枝长度,这从机械形态学角度来说,有利于通过降低机械胁迫与植株间的受力面积进而降低水动力作用于植株上的拖曳力[27, 37, 39, 91],但是矮小植株和较少较短的分枝也会显著降低植株获取可利用光的能力[24, 91]和有性繁殖能力[110-111],而分枝数、分枝长度和总分枝生物量均与无性繁殖体间呈显著正相关[6, 60-61, 91],因而富营养化生境生长的矮小、分枝少、分枝短的植株的适合度较低[25, 91, 103].而受富营养化显著影响的代谢方面,尤其是植株体内较低的碳水化合物含量会显著降低植株对寒冷、低氧、虫害等其他胁迫的耐受能力[1, 60, 96, 107],进而不利于植物的生存和繁殖,即适合度的降低.
4 研究展望 4.1 富营养化生境其他因素对水生植物生物力学性能的可能性影响富营养化水体除具有富营养化底泥、水体高浓度氮磷和可利用光缺乏3大主要特征外,还存在因大量藻类生长繁殖释放的藻毒素等化学物质、水体低溶解氧、底泥含高浓度硫化物等其他因素,这些因素积累到一定程度也会显著影响水生植物的生长、形态、组织结构和代谢等[70, 112].其中,微囊藻毒素是太湖水华期主要的毒素,野外调查研究发现太湖蓝藻水华暴发区的优势水生植物(竹叶眼子菜和荇菜)叶、茎中淀粉、可溶性糖和蔗糖含量均大幅度低于清水区的含量,微囊藻毒素的毒性胁迫还能导致挺水植物菰和芦苇体内的糖类物质减少[112];已有实验研究发现微囊藻毒素能导致植物蔗糖代谢失衡、光合作用受抑制以及叶的坏死[113-114].除了藻毒素以外,低氧与竹叶眼子菜和荇菜不同器官的碳氮代谢平衡指数呈显著负相关,这表明在水华高发的夏季,植物的碳氮代谢平衡受到低氧的胁迫[112].而硫化物也能毒害水生植物,导致芦苇植物组织学结构改变[115]、野外植株生长矮小[116]以及限制种群的生存和扩展[109],室内实验还发现硫化物能导致植物生长缓慢以及氮的吸收降低[117-118].
鉴于水生植物生物力学与形态、生物量分配、化学物质含量及组织结构的密切相关性(详见3.2节),我们推测藻毒素、低溶解氧和高浓度硫化物等富营养化生境中次因素也会在一定程度上通过改变水生植物的形态、生物量分配、组织结构和化学物质含量等影响植株的生物力学性能及其受到的机械作用力,进一步影响植株的机械损伤类型和程度.但这需要进一步的室内实验和野外实验综合研究证实.
4.2 富营养化水体中各因子间对水生植物生物力学性能的可能性影响生态系统是多因子共同作用的综合系统,每个因子并不是孤立、单独存在的,某项因子总与其他因子相互联系、相互制约.不仅水体富营养化会引发底泥富营养化、水体氮磷浓度升高、可利用光降低、藻毒素产生、溶解氧浓度降低和硫化物浓度升高等一系列因素的改变,对水生植物产生直接机械损伤的波浪、水流、船舶以及食草动物等水生境的机械胁迫,在对根生水生植物产生机械作用力[36, 41-43]的同时,也会促进水气界面气体交换、水泥界面氮磷释放和底泥再悬浮等,从而在一定程度上增加水体溶解氧、二氧化碳和氮磷等营养物质浓度、减少可利用光[119-120],这无疑进一步加剧了水体富营养化对水生生物的胁迫作用.已有部分研究表明波浪和营养、营养和光照间对水生植物的形态、代谢、生物力学性能等方面的影响存在一定的相互作用[22, 63, 107].但目前研究多集中在单一或少数物种对两三个因素在实验室条件下的短期响应,湖泊、河流、海岸带等自然水体中多个水生植物物种甚至群落对多重因子的综合的、长期的响应还缺乏深入系统了解.
综上所述,水生植物生物力学性能不仅直接受到波浪、流速和食草动物等机械胁迫的影响,也间接受到富营养化生境富营养底泥、水体的高浓度氮磷和低可利用光3大主要特征因素的影响,且具有一定的协同作用;水生植物生物力学性能与植株的生长、形态、生物量分配、组织结构、代谢等方面密切相关,表明其还可能受到对这些方面有显著影响的富营养化生境中次因素,如藻毒素和高浓度硫化物等的影响;考虑到机械胁迫在一定程度上会加剧水体富营养化带来的各变化因子的变化程度,水生植物更易发生机械损伤.此外,水生植物的生物力学性能如果不足以抵抗随着全球气候变化引发的异常降雨情势和水利工程调控的水文节律带来的水生植物在水陆生生境不断更替的机械胁迫作用力,则会造成植株断枝、拔根等机械损伤,这不仅会降低植株利用资源和物质合成、耐受能力,也会严重影响机械断枝的扩散和生根定植能力.总之,目前研究表明水生植物的生物力学性能在水体富营养化引发的水生植物衰退中具有非常重要作用,但其生物力学机理还需要长期的、系统的、多重因子综合作用的、多种的、群落的自然水体和室内实验相结合的研究来探讨总结.
| [1] |
Cao T, Xie P, Ni LY et al. Carbon and nitrogen metabolism of an eutrophication tolerative macrophyte, Potamogeton crispus, under NH4+ stress and low light availability. Environmental and Experimental Botany, 2009, 68: 74-78. DOI:10.1016/j.envexpbot.2008.10.004 |
| [2] |
Xie Y, An S, Yao X et al. Short-time response in root morphology of Vallisneria natans to sediment type and water-column nutrient. Aquatic Botany, 2005, 81: 85-96. DOI:10.1016/j.aquabot.2004.12.001 |
| [3] |
Xie Y, Luo W, Ren B et al. Morphological and physiological responses to sediment type and light availability in roots of the submersed plant Myriophyllum spicatum. Annals of Botany, 2007, 100: 1517-1523. DOI:10.1093/aob/mcm236 |
| [4] |
Ni LY. Growth of Potamogeton maackianus under low-light stress in eutrophic water. Journal of Freshwater Ecology, 2001, 16: 249-256. DOI:10.1080/02705060.2001.9663809 |
| [5] |
Cao T, Ni L, Xie P et al. Effects of moderate ammonium enrichment on three submersed macrophytes under contrasting light availability. Freshwater Biology, 2011, 56: 1620-1629. DOI:10.1111/j.1365-2427.2011.02601.x |
| [6] |
Xiong HF, Tan QL, Hu CX. Structural and metabolic responses of Ceratophyllum demersum to eutrophic conditions. African Journal of Biotechnology, 2010, 35(9): 5722-5729. |
| [7] |
Jupp BP, Spence DHN. Limitation on macrophytes in an eutrophic lake, Loch Leven. Journal of Ecology, 1977, 65: 175-186. DOI:10.2307/2259072 |
| [8] |
Phillips GL, Eminson DF, Moss B. Mechanism to account for macrophyte decline in progressively eutrophicated freshwaters. Aquatic Botany, 1978, 4: 103-126. DOI:10.1016/0304-3770(78)90012-8 |
| [9] |
Waycott M, Duarte CM, Carruthers TJB et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. PNAS, 2009, 106(30): 12377-12381. DOI:10.1073/pnas.0905620106 |
| [10] |
Gaylord B, Denny M. Flow and flexibility. I. effects of size, shape and stiffness in determining wave forces on the stipitate kelps Eisenia arborea and Pterygophora californica. Antiquité Classique Revue Interuniversitaire Detudes Classiques, 1997, 65(24): 415-416. |
| [11] |
Koehl MA. Ecological biomechanics of benthic organisms:Life history, mechanical design and temporal patterns of mechanical stress. Journal of Experimental Biology, 1999, 202: 3469-3476. |
| [12] |
Bouma TJ, De Vries MB, Low E et al. Trade-offs related to ecosystem engineering:A case study on stiffness of emerging macrophytes. Ecology, 2005, 86: 2187-2199. DOI:10.1890/04-1588 |
| [13] |
Niklas KJ, Spatz HC, Vincent J. Plant biomechanics:an overview and prospectus. American Journal of Botany, 2006, 93(10): 1369-1378. DOI:10.3732/ajb.93.10.1369 |
| [14] |
Demes KW, Carrington E, Gosline J et al. Variation in anatomical and material properties explains differences in hydrodynamic performances of foliose red macroalgae (Rhodophyta). Journal of Phycology, 2011, 47: 1360-1367. DOI:10.1111/j.1529-8817.2011.01066.x |
| [15] |
Miler O, Albayrak I, Nikora V et al. Biomechanical properties of aquatic plants and their effects on plant-flow interactions in streams and rivers. Aquatic Sciences, 2012, 74: 31-44. DOI:10.1007/s00027-011-0188-5 |
| [16] |
Nepf HM. Flow and transport in regions with aquatic vegetation. Annual Review of Fluid Mechanics, 2012, 44: 123-142. DOI:10.1146/annurev-fluid-120710-101048 |
| [17] |
Nikora V, Cameron S, Albayrak I et al. Flow-biota interactions in aquatic systems:Scales, mechanisms and challenges. In:Rodi W, Uhlmann M eds. Environmental fluid mechanics. Boca Raton: CRC Press, 2012, 217-235.
|
| [18] |
Moulia B. Plant biomechanics and mechanobiology are convergent paths to flourishing interdisciplinary research. Journal of Experimental Botany, 2013, 64(15): 4617-33. DOI:10.1093/jxb/ert320 |
| [19] |
Henry PY. Bending properties of a macroalga:adaptation of peirce's cantilever test for in situ measurements of Laminaria digitata (Laminariaceae). American Journal of Botany, 2014, 101(6): 23-31. DOI:10.3732/ajb.1400163 |
| [20] |
Zhu GR, Zhang M, Cao T et al. Associations between the morphology and biomechanical properties of submerged macrophytes:implications for its survival and distribution in lake Erhai. Environmental Earth Sciences, 2015, 74(5): 3907-3916. DOI:10.1007/s12665-015-4267-0 |
| [21] |
Lamberti-Raverot B, Puijalon S. Nutrient enrichment affects the mechanical resistance of aquatic plants. Journal of Experimental Botany, 2012, 63(17): 6115-6123. DOI:10.1093/jxb/ers268 |
| [22] |
La Nafie YA, de los Santos CB, Brun FG et al. Biomechanical response of two fast-growing tropical seagrass species subjected to in situ shading and sediment fertilization. Journal of Experimental Marine Biology & Ecology, 2013, 446(3): 186-193. DOI:10.1016/j.jembe.2013.05.020 |
| [23] |
Zhu GR, Zhang M, Cao T et al. Effects of sediment type on stem mechanical properties of the submerged macrophyte Hydrilla verticillata (L. f.) Royle. Fresenius Environmental Bulletin, 2012, 21: 468-474. |
| [24] |
Zhu GR, Cao T, Zhang M et al. Fertile sediment and ammonium enrichment decrease the growth and biomechanical strength of submersed macrophyte Myriophyllum spicatum in an experimen. Hydrobiologia, 2014, 727: 109-120. DOI:10.1007/s10750-013-1792-2 |
| [25] |
Zhu Guorong. Studies on the effects of eutrophication and floods on the biomechanical characteristics of aquatic macrophytes[Dissertation]. Wuhan:Institute of Hydrobiology, CAS, 2012. [祝国荣. 富营养化和洪水对水生植物的生物力学特征的影响研究[学位论文]. 武汉: 中国科学院水生生物研究所, 2012. http://d.wanfangdata.com.cn/Thesis_Y2250081.aspx ]
|
| [26] |
Schutten J, Dainty J, Davy AJ. Root anchorage and its significance for submersed plants in shallow lakes. Journal of Ecology, 2005, 93: 556-571. DOI:10.1111/jec.2005.93.issue-3 |
| [27] |
Puijalon S, Léna JP, Rivière N et al. Phenotypic plasticity in response to mechanical stress:hydrodynamic performance and fitness of four aquatic plant species. New Phytologist, 2008, 177: 907-917. DOI:10.1111/nph.2008.177.issue-4 |
| [28] |
Nikora V. Hydrodynamics of aquatic ecosystems:an interface between ecology, biomechanics and environmental fluid mechanics. River Research and Applications, 2010, 26: 367-384. DOI:10.1002/rra.1291 |
| [29] |
Dawson FH, Robinson WN. Submersedmacrophytes and the hydraulic roughness of a lowland chalkstream. Verhandlungen des Internationalen Verein Limnologie, 1984, 22: 1944-1948. |
| [30] |
Usherwood JR, Ennos AR, Ball DJ. Mechanical and anatomical adaptations in terrestrial and aquatic buttercups to their respective environments. Journal of Experimental Botany, 1997, 312: 1469-1475. DOI:10.1093/jxb/48.7.1469 |
| [31] |
Denny M. Extreme drag forces and the survival of wind-and water-swept organisms. Journal of Experimental Biology, 1994, 194(1): 97-115. |
| [32] |
Denny M, Gaylord B. The mechanics of wave-swept algae. Journal of Experimental Biology, 2002, 205: 1355-1362. |
| [33] |
Bociag K, Galka A, Lazarewicz T et al. Mechanical strength of stems in aquatic macrophytes. Acta Societatis Botanicorum Poloniae, 2009, 78: 181-187. DOI:10.5586/asbp.2009.022 |
| [34] |
Keddy PA. Quantifying within-lake gradients of wave energy:interrelationships of wave energy, substrate particle size and shoreline plants in Axe Lake, Ontario. Aquatic Botany, 1982, 14: 41-58. DOI:10.1016/0304-3770(82)90085-7 |
| [35] |
Hamilton DP, Mitchell SF. Wave-induced shear stresses, plant nutrients and chlorophyll in seven shallow lakes. Freshwater Biology, 1997, 38: 159-168. DOI:10.1046/j.1365-2427.1997.00202.x |
| [36] |
Gaylord B. Detailing agents of physical disturbance:wave-induced velocities and accelerations on a rocky shore. Journal of Experimental Marine Biology and Ecology, 1999, 239(1): 85-124. DOI:10.1016/S0022-0981(99)00031-3 |
| [37] |
Schutten J, Dainty J, Davy AJ. Wave-induced hydraulic forces on submersed aquatic plants in shallow lakes. Annals of Botany, 2004, 93: 333-341. DOI:10.1093/aob/mch043 |
| [38] |
Puijalon S, Bouma TJ, Douady CJ et al. Plant resistance to mechanical stress:evidence of an avoidance-tolerance tradeoff. New Phytologist, 2011, 191: 1141-1149. DOI:10.1111/j.1469-8137.2011.03763.x |
| [39] |
Schutten J, Davy AJ. Predicting hydraulic forces on submerged macrophytes from current velocity, biomass and morphology. Oecologia, 2000, 123: 445-452. DOI:10.1007/s004420000348 |
| [40] |
Albayrak I, Nikora V, Miler O et al. Flow-plant interactions at a leaf scale:effects of leaf shape, serration, roughness and flexural rigidity. Aquatic Sciences, 2012, 74: 267-286. DOI:10.1007/s00027-011-0220-9 |
| [41] |
Sand-Jensen K. Drag and reconfiguration of freshwatermacrophytes. Freshwater Biology, 2003, 48: 271-283. DOI:10.1046/j.1365-2427.2003.00998.x |
| [42] |
Vogel S. Life in moving fluids:the physical biology of flow. 2nd Edn. Princeton NJ: Princeton University Press, 1994.
|
| [43] |
Denny MW. Biology and the mechanics of the wave-swept environment. Princeton: Princeton University Press, 1988.
|
| [44] |
Niklas KJ. The evolution of plant body plans-a biomechanical perspective. Annals of Botany, 2000, 85(4): 411-438. DOI:10.1006/anbo.1999.1100 |
| [45] |
Boller ML, Carrington E. In situ, measurements of hydrodynamic forces imposed on Chondrus crispus, stackhouse. Journal of Experimental Marine Biology & Ecology, 2006, 337(2): 159-170. DOI:10.1016/j.jembe.2006.06.011 |
| [46] |
Koehl MAR. How do benthic organisms withstand moving water?. American Zoologist, 1984, 24(1): 57-70. DOI:10.1093/icb/24.1.57 |
| [47] |
Denny MW, Gaylord BP, Cowen EA. Flow and flexibility. Ⅱ. The roles of size and shape in determining wave forces on the bull kelp Nereocystis luetkeana. The Journal of Experimental Biolog, 1997, 200(24): 3165-3183. |
| [48] |
Hamann E, Puijalon S. Biomechanical responses of aquatic plants to aerial conditions. Annals of Botany, 2013, 112: 1869-1878. DOI:10.1093/aob/mct221 |
| [49] |
Coops H, Van der Velde G. Effects of waves on helophyte stands, mechanical characteristics of stems of Phragmites Phragmites australis and Scirpus lacustris. Aquatic Botany, 1996, 53: 175-185. DOI:10.1016/0304-3770(96)01026-1 |
| [50] |
Etnier SA, Villani PJ. Differences in mechanical and structural properties of surface and aerial petioles of the aquatic plant Nymphaea odorata subsp. tuberosa (Nymphaeaceae). American Journal of Botany, 2007, 94: 1067-1072. DOI:10.3732/ajb.94.7.1067 |
| [51] |
Maltchik L, Rolon AS, Schott P. Effects of hydrological variation on the aquatic plant community in a floodplain palustrine wetland of southern brazil. Limnology, 2007, 8(1): 23-28. DOI:10.1007/s10201-006-0192-y |
| [52] |
Hussner A, Meyer C, Busch J. Influence of water level on growth and root system development of Myriophyllum aquaticum (Vell.) Verdcour. Weed Research, 2009, 49(1): 73-80. DOI:10.1111/wre.2009.49.issue-1 |
| [53] |
Heidbüchel P, Kuntz K, Hussner A. Alien aquatic plants do not have higher fragmentation rates than native species:A field study from the River Erft. Aquatic Sciences, 2016, 1-11. DOI:10.1007/s00027-016-0468-1 |
| [54] |
IPCC. Summary for policymakers. In:Field CB, Barros VR, Dokken DJ et al eds. Climate change 2014:Impacts, adaptation, and vulnerability. Contribution of Working Group Ⅱ to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press, 2014, 1-32.
|
| [55] |
Najjar RG, Pyke CR, Adams MB et al. Potential climate-change impacts on the Chesapeake bay. Estuarine Coastal & Shelf Science, 2010, 86(1): 1-20. DOI:10.1016/j.ecss.2009.09.026 |
| [56] |
Young IR, Zieger S, Babanin AV. Global trends in wind speed and wave height. Science, 2011, 332: 451-455. DOI:10.1126/science.1197219 |
| [57] |
Dudgeon SR, Johnson AS. Thick versus thin:thallus morphology and tissue mechanics influence differential drag and dislodgement of co-dominant seaweeds. Journal of Experimental Marine Biology and Ecology, 1992, 165: 23-43. DOI:10.1016/0022-0981(92)90287-K |
| [58] |
Mony C, Puijalon S, Bornette G. Response of clonal plants to disturbances:does resprouting pattern determine ecological niche. Folia Geobotanica, 2011, 46: 155-164. DOI:10.1007/s12224-010-9095-0 |
| [59] |
Zhu GR, Li W, Zhang M et al. Adaptation of submerged macrophytes to both water depth and flood intensity as revealed by their mechanical resistance. Hydrobiologia, 2012, 696(1): 77-93. DOI:10.1007/s10750-012-1185-y |
| [60] |
Kimbel JC. Factors influencing potential intralake colonization by Myriophyllum spicatum L. Aquatic Botany, 1982, 14: 295-307. DOI:10.1016/0304-3770(82)90104-8 |
| [61] |
Smith DH, Madsen JD, Dickson KL et al. Nutrient effects on autofragmentation of Myriophyllum spicatum. Aquatic Botany, 2002, 74: 1-17. DOI:10.1016/S0304-3770(02)00023-2 |
| [62] |
Ennos AR. The scaling of root anchorage. Journal of Theoretical Biology, 1993, 161(1): 61-75. DOI:10.1006/jtbi.1993.1040 |
| [63] |
La Nafie YA, de los Santos CB, Brun FG et al. Waves and high nutrient loads jointly decrease survival and separately affect morphological and biomechanical properties in the seagrass Zostera noltii. Limnology & Oceanography, 2012, 57(6): 1664-1672. |
| [64] |
Brewer CA, Parker M. Adaptations of macrophytes to life in moving water:upslope limits and mechanical properties of stems. Hydrobiologia, 1990, 194: 133-142. DOI:10.1007/BF00028414 |
| [65] |
Koehl MAR. Seaweeds in moving water:form and mechanical function. In:Givnish T ed. On the economy of plant form and function. Cambridge: Cambridge University Press, 1986, 603-634.
|
| [66] |
Linkohr BI, Williamson LC, Fitter AH et al. Nitrogen and phosphorus availability and distribution have different effects on root system architecture of Arabidopsis. The Plant Journal, 2002, 29: 751-760. DOI:10.1046/j.1365-313X.2002.01251.x |
| [67] |
Dupuy L, Fourcaud T, Stokes A. A numerical investigation into the influence of soil type and root architecture on tree anchorage. Plant and Soil, 2005, 278(1/2): 119-134. |
| [68] |
Burkholder JM, Tomasko DA, Touchette BW. Seagrasses and eutrophication. Journal of Experimental Marine Biology and Ecology, 2007, 350: 46-72. DOI:10.1016/j.jembe.2007.06.024 |
| [69] |
Cabaço S, Machas R, Vieira V et al. Impacts of urban wastewater discharge on seagrass meadows (Zostera noltii). Estuarine, Coastal and Shelf Science, 2008, 78: 1-13. DOI:10.1016/j.ecss.2007.11.005 |
| [70] |
Sand-Jensen K, Borum J. Interactions among phytoplankton, periphyton, and macrophytes in temperate freshwaters and estuaries. Aquatic Botany, 1991, 41: 137-175. DOI:10.1016/0304-3770(91)90042-4 |
| [71] |
Ni LY. Stress of fertile sediment on the growth of submersed macrophytes in eutrophic waters. Acta Hydrobiologica Sinica, 2001, 25: 399-405. |
| [72] |
Niklas KJ. Effects of vibration on mechanical properties and biomass allocation pattern of Capsella bursapastoris (Crucife-rae). Annals of Botany, 1998, 82: 147-156. DOI:10.1006/anbo.1998.0658 |
| [73] |
Burylo M, Rey F, Roumet C et al. Linking plant morphological traits to uprooting resistance in eroded marly lands (Southern Alps, France). Plant and Soil, 2009, 324: 31-42. DOI:10.1007/s11104-009-9920-5 |
| [74] |
Crook MJ, Ennos AR. The anchorage mechanics of deeprooted larch, Larix europea×L. japonica. Journal of Experimental Botany, 1996, 47(10): 1509-1517. DOI:10.1093/jxb/47.10.1509 |
| [75] |
Cucchi V, Meredieu C, Stokes A et al. Root anchorage of inner and edge trees in stands of Maritime pine (Pinus pinaster Ait.) growing in different podzolic soil conditions.. Trees, 2004, 18(4): 460-466. DOI:10.1007/s00468-004-0330-2 |
| [76] |
Mickovski SB, Ennos AR. A morphological and mechanical study of the root systems of suppressed crown Scots pine Pinus sylvestris. Trees (Berl), 2002, 16: 274-280. DOI:10.1007/s00468-002-0177-3 |
| [77] |
Nicoll BC, Gardiner BA, Rayner B et al. Anchorage of coniferous trees in relation to species, soil type, and rooting depth. Canadian Journal of Forest Research, 2006, 36: 1871-1883. DOI:10.1139/X06-072 |
| [78] |
Karrenberg S, Blaser S, Edwards PJ et al. Root anchorage of saplings and cuttings of woody pioneer species in a riparian environment. Functional Ecology, 2003, 17: 170-177. DOI:10.1046/j.1365-2435.2003.00709.x |
| [79] |
Khuder H, Stokes A, Danjon F et al. Is it possible to manipulate root anchorage in young trees?. Plant Soil, 2007, 294: 87-102. DOI:10.1007/s11104-007-9232-6 |
| [80] |
Crook MJ, Ennos AR. The increase in anchorage with tree size of the tropical tap rooted tree Mallotus wrayi, King (Euphorbiaceae). Annals of Botany, 1998, 82: 291-296. DOI:10.1006/anbo.1998.0678 |
| [81] |
Ennos AR, Crook MJ, Grimshaw C. A comparative study of the anchorage systems of himalayan balsam Impatiens glandulifera and mature sunfower Helianthus annuus. Journal of Experimental Botany, 1993, 44: 133-146. DOI:10.1093/jxb/44.1.133 |
| [82] |
Goodman AM, Crook MJ, Ennos AR. Ennos AR. Anchorage mechanics of the tap root system of winter-sown oilseed rape (Brassica napus L.). Annals of Botany, 2001, 87: 397-404. DOI:10.1006/anbo.2000.1347 |
| [83] |
Toukura Y, Devee E, Hongo A. Uprooting and shearing resistances in the seedlings of four weedy species. Weed Biology and Management, 2006, 6: 35-43. DOI:10.1111/j.1445-6664.2006.00192.x |
| [84] |
Bailey PHJ, Currey JD, Fitter AH. The role of root system architecture and root hairs in promoting anchorage against uprooting forces in Allium cepa and root mutants of Arabidopsis thaliana. Journal of Experimental Botany, 2002, 53: 333-340. DOI:10.1093/jexbot/53.367.333 |
| [85] |
Crook MJ, Ennos AR. The mechanics of root lodging in winter wheat Triticum aestivum L. Journal of Experimental Botany, 1993, 44: 1219-1224. DOI:10.1093/jxb/44.7.1219 |
| [86] |
Ennos AR, Crook MJ, Grimshaw C. The anchorage mechanics of maize Zea mays. Journal of Experimental Botany, 1993, 44: 147-153. DOI:10.1093/jxb/44.1.147 |
| [87] |
Mickovski SB, van Beek LPH, Salin F. Uprooting resistance of vetiver grass (Vetiveria zizanioides). Plant Soil, 2005, 278: 33-41. DOI:10.1007/s11104-005-2379-0 |
| [88] |
Stokes A, Lucas A, Jouneau L. Plant biomechanical strategies in response to frequent disturbance:uprooting of Phyllostachys nidularia (Poaceae) growing on landslide prone slopes in Sichuan, China. American Journal of Botany, 2007, 94(7): 1129-1136. DOI:10.3732/ajb.94.7.1129 |
| [89] |
Loades KW, Bengough AG, Bransby MF et al. Effect of root age on the biomechanics of seminal and nodal roots of barley (Hordeum vulgare L.) in contrasting soil environments. Plant and Soil, 2015, 395: 253-261. DOI:10.1007/s11104-015-2560-z |
| [90] |
Chimungu JG, Loades KW, Lynch JP. Root anatomical phenes predict root penetration ability and biomechanical properties in maize (Zea Mays). Journal of Experimental Botany, 2015, 66(11): 3151-3162. DOI:10.1093/jxb/erv121 |
| [91] |
Puijalon S, Bornette G. Morphological variation of two taxonomically distant plant species along a natural flow velocity gradient. New Phytologist, 2004, 163(3): 651-660. DOI:10.1111/j.1469-8137.2004.01135.x |
| [92] |
Kheiralla KA, Mehdi EE, Dawood RA. Evaluation of some wheat cultivars for traits related to lodging resistance under different levels of nitrogen. Assiut Journal of Agricultural Sciences, 1993, 24: 257-271. |
| [93] |
Kaack K, Schwarz KU. Morphological and mechanical properties of Miscanthus in relation to harvesting, lodging, and growth conditions. Industrial Crops and Products, 2001, 14: 145-154. DOI:10.1016/S0926-6690(01)00078-4 |
| [94] |
Shimono H, Okada M, Yamakawa Y et al. Lodging in rice can be alleviated by atmospheric CO2 enrichment. Agriculture, Ecosystems and Environment, 2007, 118: 223-230. DOI:10.1016/j.agee.2006.05.015 |
| [95] |
Green EP, Short FT. World atlas of seagrasses. California: University of California Press, 2003.
|
| [96] |
Cao T, Xie P, Ni LY et al. The role of NH4+ toxicity in the decline of the submersed macrophyte Vallisneria natans in lakes of the Yangtze River basin. China. Marine and Freshwater Research, 2007, 58: 581-587. DOI:10.1071/MF06090 |
| [97] |
Nimptsch J, Pflugmacher S. Ammonium triggers the promotion of oxidative stress in the aquatic macrophyte Myriophyllum mattogrossense. Chemosphere, 2007, 66: 708-714. DOI:10.1016/j.chemosphere.2006.07.064 |
| [98] |
Brun FG, Hernandez I, Vergara JJ et al. Assessing the toxicity of ammonium pulses to the survival and growth of Zostera noltii. Marine Ecology Progress Series, 2002, 225: 177-187. DOI:10.3354/meps225177 |
| [99] |
Brun F, Olivé I, Malta E et al. Increased vulnerability of Zostera noltii to stress caused by low light and elevated ammonium levels under phosphate deficiency. Marine Ecology Progress Series, 2008, 365: 67-75. DOI:10.3354/meps07512 |
| [100] |
Olsen S, Chan FY, Li W et al. Strong impact of nitrogen loading on submerged macrophytes and algae:A long-term mesocosm experiment in a shallow Chinese lake. Freshwater Biology, 2015, 60: 1525-1536. DOI:10.1111/fwb.12585 |
| [101] |
Carrington E. Drag and dislodgment of an intertidal macroalga:consequences of morphological variation in Mastocarpus papillatus kützing. Journal of Experimental Marine Biology & Ecology, 1990, 139(3): 185-200. DOI:10.1016/0022-0981(90)90146-4 |
| [102] |
Shaughnessy FJ, Bell EC, Wreede RD. Consequences of morphology and tissue strength to blade survivorship of two close-ly related rhodophyta species. Marine Ecology Progress, 1996, 136(1/2/3): 257-266. DOI:10.3354/meps136257 |
| [103] |
Riis T, Madsen TV, Sennels RSH. Regeneration, colonisation and growth rates of all fragments in four common stream plants. Aquatic Botany, 2009, 90: 209-212. DOI:10.1016/j.aquabot.2008.08.005 |
| [104] |
Xie D, Yu D. Size-related auto-fragment production and carbohydrate storage in auto-fragment of Myriophyllum spicatum L. in response to sediment nutrient and plant density. Hydrobiologia, 2011, 658(1): 221-231. DOI:10.1007/s10750-010-0475-5 |
| [105] |
Doyle RD, Grodowitz MJ, Smart RM et al. Impact of herbivory by Hydrellia pakistanae (Diptera:Ephydriadae) on growth and photosynthetic potential of Hydrilla verticillata. Biological Control, 2002, 24(3): 221-229. DOI:10.1016/S1049-9644(02)00024-5 |
| [106] |
Ye Bibi. Impact to water environment with emergent plants from Erhai lakeshore and the parameters of harvest[Dissertation]. Hefei:Anhui Agricultural University, 2011. [叶碧碧. 洱海湖滨带挺水植物对湖体水环境影响及收割参数研究[学位论文]. 合肥: 安徽农业大学, 2011]. http://cdmd.cnki.com.cn/Article/CDMD-10364-1013161436.htm ]
|
| [107] |
Kopp BS. Effects of nitrate fertilization and shading on physiological and biomechanical properties of eelgrass (Zostera marina L.)[Dissertation]. Rhode Island:University of Rhode Island, 1999. https://www.researchgate.net/publication/36417378_Effects_of_nitrate_fertilization_and_shading_on_physiological_and_biomechanical_properties_of_eelgrass_Zostera_marina_L
|
| [108] |
Qiu Dongru, Wu Zhenbin. On the decline and restoration of submerged vegetation in eutrophic shallow lakes. J Lake Sci, 1997, 9(1): 82-88. [邱东茹, 吴振斌. 富营养化浅水湖泊沉水水生植被的衰退与恢复. 湖泊科学, 1997, 9(1): 82-88. DOI:10.18307/1997.0113] |
| [109] |
Govers LL, Brouwer JHFD, Suykerbuyk W et al. Toxic effects of increased sediment nutrient and organic matter loading on the seagrass zostera noltii. Aquatic Toxicology, 2014, 155(4): 253-260. DOI:10.1016/j.aquatox.2014.07.005 |
| [110] |
Samson DA, Werk KS. Size-dependent effects in the analysis of reproductive effort in plants. American Naturalist, 1986, 127(5): 667-680. DOI:10.1086/284512 |
| [111] |
Shipley B, Dion J. The allometry of seed production in herbaceous angiosperms. American Naturalist, 1992, 139(3): 467-483. DOI:10.1086/285339 |
| [112] |
Zhang Meng. Physio-ecological responses of aquatic macrophytes to the stresses of lake eutrophication[Dissertation]. Wuhan:Institute of Hydrobiology, CAS, 2010. [张萌. 水生植物对湖泊富营养化胁迫的生理生态学响应[学位论文]. 武汉: 中国科学院水生生物研究所, 2010. http://www.irgrid.ac.cn/handle/1471x/210342 ]
|
| [113] |
Siegl G, MacKintosh C, Stitt M. Sucrose phosphate synthetase is dephosphorylated by protein phosphatase 2A in spinach leaves. FEBS Letters, 1990, 270: 198-202. DOI:10.1016/0014-5793(90)81267-R |
| [114] |
Abe T, Lawson T, Weyers JDB et al. Microcystin-LR inhibits photosynthesis of Phaseolus vulgaris primary leaves:Implications for current spray irrigation practice. New Phytologist, 1996, 133: 651-658. DOI:10.1111/j.1469-8137.1996.tb01934.x |
| [115] |
Armstrong J, Areen ZF, Armstrong W. Phragmites die-back:Sulphide-and acetic acid-induced bud and root death, lignifications, and blockages within aeration and vascular systems. New Phytologist, 1996, 134: 601-614. DOI:10.1111/j.1469-8137.1996.tb04925.x |
| [116] |
King GM, Klug MJ, Wiegert RG et al. Relation of soil water movement and sulfide concentration to Spartina alterniflora production in a Georgia salt marsh. Science, 1982, 218: 61-63. DOI:10.1126/science.218.4567.61 |
| [117] |
Koch MS, Mendelssohn IA. Sulfide as a soil phytotoxin:differential responses in two marsh species. Journal of Ecology, 1989, 77: 565-578. DOI:10.2307/2260770 |
| [118] |
Koch MS, Mendelssohn IA, McKee KL. Mechanism for the hydrogen sulfide-induced growth limitation in wetland macrophytes. Limnology & Oceanography, 1990, 35: 399-408. DOI:10.4319/lo.1990.35.2.0399 |
| [119] |
Koch EW. Hydrodynamics, diffusin-boundary layers and photosynthesis of the seagrasses Thalassia testudium and Cymodocea nodosa. Marine Biology, 1994, 118: 767-776. DOI:10.1007/BF00347527 |
| [120] |
Madsen JD, Chambers PA, James WF et al. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia, 2001, 444: 71-84. DOI:10.1023/A:1017520800568 |
 2017, Vol. 29
2017, Vol. 29 

