(2: 中国科学院南京地理与湖泊研究所, 南京 210008)
(2: Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, P. R. China)
生态修复成为改善富营养化水体水质的一种有效方法[1].底栖动物,特别是滤食性河蚌因其可滤食浮游植物、有机碎屑等悬浮颗粒[2],被认为可增加水体透明度[3-5],改善水质,已广泛应用.如Hosper等[6]在荷兰的一些浅水湖泊的生态修复中运用了斑马贻贝(Dreissena polymorpha);在太湖蠡湖和上海金山城市沙滩人工潟湖水体的生态修复中也运用了滤食性河蚌[7-8].除滤食外,河蚌的呼吸、分泌、排泄等生理活动,也可显著增加水中营养盐浓度[9].滤食性河蚌不仅可直接分泌释放出大量溶解性氮、磷营养盐[9-10];也可通过粪便排泄等基本生理代谢活动释放营养盐[10];还可在沉积物表层形成大量富含营养物质的假粪,更易分解而增加水层中的营养盐[10-11];再者,其还能促进沉积物营养盐释放[9, 12],这些又被认为会恶化水质.如厦门筼筜湖因沙筛贝(Mytilopsis sallei)入侵,加剧了筼筜湖的富营养化[13].因此,滤水速率的快慢是滤食性河蚌对水质改善与否的关键.
滤食性河蚌的滤水速率快慢受蚌龄大小、食物多少与季节变化等因素的影响.如滤食性河蚌的滤水速率随浮游藻类浓度变化而不同,低藻浓度条件下滤食性贝类的滤水速率低下[14];河蚌滤水速率与蚌龄的大小有关,小型河蚌的滤食活动更强[15-16].因此,了解蚌龄大小、食物多少以及季节变化对河蚌滤水速率的影响,对进一步认识河蚌能否改善水质以及富营养化水体生态修复中如何优化利用滤食性河蚌具有重要意义.
本研究以背角无齿蚌(Anodonta woodiana)为对象,采用野外原位实验的方法,开展清水态和富营养化条件下幼龄和成年背角无齿蚌滤食对水质改善效果的实验,测定不同处理组水中氮、磷、总悬浮物(TSS)浓度和浮游藻类生物量(用叶绿素a(Chl.a)浓度表示)的季节变化,研究蚌龄大小、食物多少和季节变化等因素对滤水速率的影响,以期为湖泊生态系统保护和富营养化湖泊的修复提供参考.
1 材料与方法 1.1 实验材料背角无齿蚌采自广东省惠州西湖.称重前用软毛刷刷掉蚌壳表面的附着生物,湖水洗净,用吸水纸去除蚌壳表面水分.实验用水取自广东省惠州西湖,用13#浮游生物网滤除湖水中的大型浮游动物.
实验用桶为聚乙烯塑料材质,容积120 L,具底,底部直径×上部直径×高≈45 cm×57 cm×61 cm.
1.2 实验方法将实验用桶(固定于惠州西湖中)中注入60 L用13#浮游生物网滤除浮游动物的惠州西湖湖水,分为幼龄蚌组、成年蚌组以及没有蚌的对照组,每组4次重复.幼龄蚌组每桶放置1只幼龄蚌(壳长=78.0±3.7 mm,鲜重=54.6±4.7 g);成年蚌组每桶放置1只成年蚌(壳长=110.1±1.6 mm,鲜重=136.3±8.4 g).实验在春、夏、秋、冬季节选择晴朗天气分别在惠州西湖生态修复示范区的清水态水体和未修复的富营养化水体平湖进行,每次实验10:00开始,14:00结束.
1.3 采样与分析实验开始和结束时分别用1000 ml的聚乙烯塑料瓶采集水下10~20 cm处桶内水样和桶外湖泊水样,置于加冰的采集箱待测.该水样用于分析总氮(TN)、总磷(TP)、铵态氮(NH4+-N)、总悬浮物(TSS)浓度和Chl.a浓度. Chl.a浓度采用丙酮萃取分光光度法测定[17];TN浓度采用碱性过硫酸钾紫外分光光度法测定,TP浓度采用钼酸铵分光光度法进行测定[18];NH4+-N浓度采用纳氏试剂比色法进行测定[19]. TSS浓度采用Whatman GF/C纤维滤膜抽滤后105℃烘干称重的方法[20]进行测定.
背角无齿蚌滤水速率计算公式为[21]:
| $ FR = V/\left( {W \cdot t} \right)[(\ln {C_0} - \ln {C_t}) - (\ln {C_0}' - \ln {C_t}')] $ | (1) |
式中,FR为滤水速率(L/(g·h)),V为实验桶中水的体积(L),W为蚌的质量(g),t为时间(h),C0和Ct分别为实验组实验开始和t时间悬浮有机物浓度(mg/L),C0'和Ct'分别为对照组实验开始和t时间悬浮有机物浓度(mg/L).
1.4 数据统计分析所有实验数据采用SPSS 18.0软件进行统计分析,各处理组随季节性变化的差异采用重复测量方差分析(RM-ANOVAs),组间差异采用单因素方差分析(One-way ANOVA). *表示与对照组差异显著(P < 0.05).
2 结果 2.1 营养盐清水态水体中,除秋季的幼龄蚌组外,春、夏、秋、冬季各蚌处理组TP浓度均高于对照组(P < 0.05);但各季节的幼龄蚌组与成年蚌组间TP浓度差异不显著(P>0.05).富营养化水体中,幼龄蚌组的秋、冬季TP浓度显著低于对照组(P < 0.05);成年蚌组的春、冬季TP浓度也显著低于对照组(P < 0.05);其他季节各处理组组间差异不显著(P>0.05)(图 1).
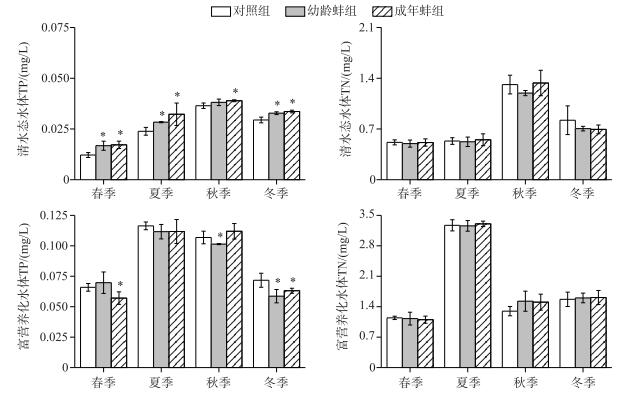
|
图 1 不同处理组TP和TN浓度 Fig.1 TP and TN concentrations in different treatments |
不管在清水态水体还是富营养化水体中,各处理组TN浓度差异不显著(P>0.05);但在清水态水体的春、秋季,成年蚌组NH4+-N浓度显著高于对照组(P < 0.05);幼龄蚌组的NH4+-N浓度在春季(0.18±0.02 mg/L)也显著高于对照组(0.13±0.01 mg/L)(P < 0.05)(图 2).
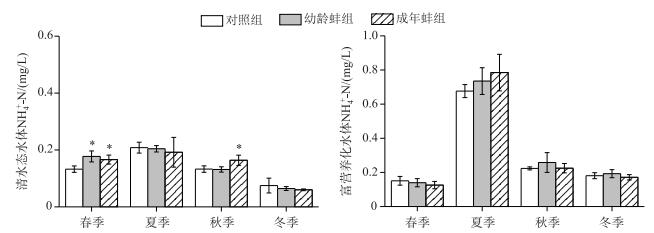
|
图 2 不同处理组NH4+-N浓度 Fig.2 Ammonium nitrogen concentrations in different treatments |
清水态水体中,幼龄蚌组和成年蚌组水中TSS浓度和Chl.a浓度与对照组相比差异均不显著(P>0.05);富营养化水体中,春、冬季的幼龄蚌组和成年蚌组水体TSS浓度均低于对照组(P < 0.05),但幼龄蚌组与成年蚌组之间的差异不显著(P>0.05);春季对照组TSS浓度为10.74±1.54 mg/L,幼龄蚌组为7.92±0.73 mg/L,成年蚌组为8.16±0.95 mg/L;冬季对照组TSS浓度为16.11±0.51 mg/L,幼龄蚌组为12.80±1.37 mg/L,成年蚌组为12.82±1.08 mg/L.夏、秋两季蚌处理组与对照组之间差异不显著(P>0.05)(图 3).

|
图 3 不同处理组TSS和Chl.a浓度 Fig.3 TSS and Chl.a concentrations in different treatments |
富营养化水体中,夏、秋和冬季幼龄蚌组浮游藻类Chl.a浓度分别为31.14±1.78、41.9±0.34和10.73±0.58 μg/L,分别低于对照组的38.91±3.29、50.03±5.54和18.85±1.50 μg/L和成年蚌组的40.08±5.39、45.24±1.23和21.97±4.59 μg/L(P < 0.05),但春季幼龄蚌组与对照组差异不显著(P>0.05);成年蚌组与对照组间差异各季节均不显著(P>0.05);浮游藻类Chl.a浓度随季节变换差异显著(RM-ANOVAs, P < 0.05),夏、秋季显著高于春、冬季(P < 0.05)(图 3).
2.3 滤水速率富营养化水体中,幼龄蚌组与成年蚌组的滤水速率随季节变化差异显著(RM-ANOVAs, P < 0.05),其中,春季最大,冬季次之,夏季最小.幼龄蚌组的滤水速率春季为0.132±0.018 L/(g·h),冬季为0.109±0 L/(g·h);成年蚌组的滤水速率春季为0.035±0.003 L/(g·h),冬季为0.019±0.004 L/(g·h);幼龄蚌组的滤水速率显著高于成年蚌组(P < 0.05).夏季幼龄蚌组和成年蚌组的滤水速率均低.
而在清水态水体中,水层中悬浮颗粒物和浮游藻类浓度低,且与对照组相比差异不显著,各处理组背角无齿蚌的滤水速率几乎为0.
3 结论与讨论 3.1 背角无齿蚌对不同营养状态水体水质的影响本研究显示,富营养化水体中,春、冬两季幼龄蚌组和成年蚌组TSS浓度均低于对照组,且夏、秋、冬季幼龄蚌组浮游藻类Chl.a浓度也低于对照组,表明背角无齿蚌降低了TSS浓度和浮游藻类密度.另外,富营养化水体中,幼龄蚌组的TP浓度在秋、冬季低于对照组;成年蚌组的TP浓度在春、冬季也低于对照组,表明背角无齿蚌降低了富营养化水体中TP浓度.水中TSS浓度、浮游藻类Chl.a浓度和TP浓度的降低,不仅有利于改善水质,也有利于提高水体透明度,从而改善浅水水体底部光照条件,为底栖植物(沉水植物和底栖藻类)的生长进一步创造条件.
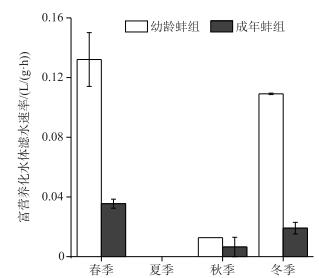
|
图 4 富营养化水体背角无齿蚌滤水速率 Fig.4 Filtration rates of mussels in eutrophic water conditions |
滤食性贝类不仅能通过滤食作用改善水质,还可通过分泌、排泄等生理活动释放营养盐[22-23].本研究显示清水态水体中,除秋季的幼龄蚌组外,春、夏、秋、冬季各蚌处理组TP浓度均高于对照组;春、秋季,成年蚌组NH4+-N浓度高于对照组;幼龄蚌组的NH4+-N浓度也在春季高于对照组,这表明背角无齿蚌提高了清水态水体中营养盐浓度.另在清水态水体中,幼龄蚌组和成年蚌组TSS浓度和浮游藻类Chl.a浓度与对照组相比差异不显著,表明清水态水体中背角无齿蚌对TSS浓度和浮游藻类Chl.a浓度影响效果不明显.已有研究表明蚌类滤食活动因悬浮颗粒浓度而变化,食物浓度低会导致滤食活动弱,甚至停止[24].本研究中清水态水体背角无齿蚌对TSS浓度和浮游藻类Chl.a浓度影响效果不明显,可能是因为其浓度低所致.
3.2 背角无齿蚌滤水速率的季节变化温度是季节变化中最重要的生态因子之一.温度变化也影响到背角无齿蚌的滤水速率.本研究表明,富营养化水体中背角无齿蚌滤水速率春季最大,冬季次之,夏季最小,这可能是实验期间夏、秋季较高水温(分别为32.9~35.4和28.4~29.7℃)降低了背角无齿蚌的滤水速率,而春季和冬季水温分别为22.7~24.7和20.9~22.3℃.有研究显示高温可降低滤食性河蚌的滤水速率,如斑马贻贝的滤水速率在20~24℃时比28~32℃高[25-26];当温度超出适宜的范围时,会降低摄食器官的活力,从而导致滤水速率的下降[27].
3.3 蚌龄大小对水质的影响不同蚌龄背角无齿蚌的滤水速率有较大差别[2, 15-16].本研究显示富营养化水体中,春、冬两季幼龄蚌组的滤水速率显著高于成年蚌组.吴庆龙等[16]的研究也表明体重小的幼龄蚌滤水速率显著高于体重大的成年蚌.本研究富营养化水体中,幼龄蚌组浮游藻类Chl.a浓度显著低于成年蚌组,也表明幼龄蚌组滤水速率高于成年蚌组.
综上,富营养化水体中,背角无齿蚌在春、冬季节滤水速率高,可降低TSS、浮游藻类Chl.a和TP浓度,从而改善水质,且幼龄蚌组的滤水速率高于成年蚌组;因此,在富营养化水体的修复前期,可放养本地滤食性河蚌,在春季放养幼龄蚌更佳,以改善水质,提高水体透明度,为接下来的修复创造条件.在清水态水体中,背角无齿蚌滤水速率低,对水质改善效果不明显,反而提高水体TP、NH4+-N等浓度.因此,在生态修复后期的清水态水体中单独的滤食性河蚌对水质改善效果不明显.
| [1] |
Hansson LA, Annadotter H, Bergman E et al. Biomanipulation as an application of food-chain theory: Constraints, synthesis, and recommendations for temperate lakes. Ecosystems, 1998, 1(6): 558-574. DOI:10.1007/s100219900051 |
| [2] |
Horgan MJ, Mills EL. Clearance rates and filtering activity of zebra mussel (Dreissena polymorpha): Implications for freshwater lakes. Canadian Journal of Fisheries and Aquatic Sciences, 1997, 54(2): 249-255. DOI:10.1139/f96-276 |
| [3] |
Reeders HH, Bi J, De Vaate A. Zebra mussels (Dreissena polymorpha): A new perspective for water quality management. Hydrobiologia, 1990, 200(1): 437-450. |
| [4] |
Ismail NS, Dodd H, Sassoubre LM et al. Improvement of urban lake water quality by removal of Escherichia coli through the action of the bivalve Anodonta californiensis. Environmental Science & Technology, 2015, 49(3): 1664-1672. |
| [5] |
Mayer CM, Keats RA, Rudstam LG et al. Scale-dependent effects of zebra mussels on benthic invertebrates in a large eutrophic lake. Journal of the North American Benthological Society, 2002, 21(4): 616-633. DOI:10.2307/1468434 |
| [6] |
Hosper SH. Biomanipulation, new perspectives for restoration of shallow, eutrophic lakes in The Netherlands. Aquatic Ecology, 1989, 23(1): 5-10. |
| [7] |
Jiang X, Wang SH, Yang XF et al. Change in water quality and ecosystem of Lihu Lake before and after comprehensive water environmental improvement measures. Research of Environmental Sciences, 2014, 27(6): 595-601. [姜霞, 王书航, 杨小飞等. 蠡湖水环境综合整治工程实施前后水质及水生态差异. 环境科学研究, 2014, 27(6): 595-601.] |
| [8] |
Ye WJ, Chen YQ. Ecological restoration and effective assessment of an artificial lagoon in city beach of Jinshan district in Shanghai. Fisheries Science, 2014, 33(12): 794-799. [叶维钧, 陈亚瞿. 上海金山城市沙滩人工潟湖水体生态修复及效果评价. 水产科学, 2014, 33(12): 794-799.] |
| [9] |
Nogaro G, Steinman AD. Influence of ecosystem engineers on ecosystem processes is mediated by lake sediment properties. Oikos, 2014, 123(4): 500-512. DOI:10.1111/more.2014.123.issue-4 |
| [10] |
Conroy JD, Edwards WJ, Pontius RA et al. Soluble nitrogen and phosphorus excretion of exotic freshwater mussels (Dreissena spp.): potential impacts for nutrient remineralisation in western Lake Erie. Freshwater Biology, 2005, 50(7): 1146-1162. DOI:10.1111/fwb.2005.50.issue-7 |
| [11] |
Higgins SN, Vander Zanden MJ. What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecological Monographs, 2010, 80(2): 179-196. DOI:10.1890/09-1249.1 |
| [12] |
Zhang XF, Liu ZW, Jeppesen E et al. Effects of deposit-feeding tubificid worms and filter-feeding bivalves on benthic-pelagic coupling: Implications for the restoration of eutrophic shallow lakes. Water Research, 2014, 50: 135-146. DOI:10.1016/j.watres.2013.12.003 |
| [13] |
Cai LZ, Hwang JS, Dahms HU et al. Effect of the invasive bivalve Mytilopsis sallei on the macrofaunal fouling community and the environment of Yundang Lagoon, Xiamen, China. Hydrobiologia, 2014, 741(1): 101-111. DOI:10.1007/s10750-014-2012-4 |
| [14] |
Marescaux J, Falisse E, Lorquet J et al. Assessing filtration rates of exotic bivalves: dependence on algae concentration and seasonal factors. Hydrobiologia, 2016, 777(1): 1-12. DOI:10.1007/s10750-016-2767-x |
| [15] |
Ding T, Li L, Peng L et al. Studies on elimination of chlorophyll-a and ingestion rate in eutrophic water by Anodonta woodiana elliptica. Acta Hydrobiologica Sinica, 2010, 34(4): 779-786. [丁涛, 李林, 彭亮等. 背角无齿蚌摄食率及对水中叶绿素a清除能力的研究. 水生生物学报, 2010, 34(4): 779-786.] |
| [16] |
Wu QL, Chen YW, Liu ZW. Filtering capacity of Anodonta woodiana and its feeding selectivity on phytoplankton. Chinese Journal of Applied Ecology, 2005, 16(12): 2423-2427. [吴庆龙, 陈宇炜, 刘正文. 背角无齿蚌对浮游藻类的滤食选择性与滤水率研究. 应用生态学报, 2005, 16(12): 2423-2427. DOI:10.3321/j.issn:1001-9332.2005.12.040] |
| [17] |
Jespersen AM, Christoffersen K. Measurements of chlorophyll-a from phytoplankton using ethanol as extraction solvent. Archiv für Hydrobiologie, 1987, 109(3): 445-454. |
| [18] |
American Public Health Association. American Water Works Association and Water Environment Federation. Standard methods for the examination of water and wastewater, 1998: 39-42.
|
| [19] |
"Water and wastewater monitoring and analysis method" editorial board of State Environmental Protection Administration of China. Monitoring and analysis methods of water and wastewater: fourth edition. Beijing: China Environmental Science Press, 2002. [国家环境保护总局《水和废水监测分析方法》编委会. 水和废水监测分析方法:第四版. 北京: 中国环境科学出版社, 2002.]
|
| [20] |
Mei X, Zhang X, Kassam SS et al. Will the displacement of zebra mussels by quagga mussels increase water clarity in shallow lakes during summer? Results from a mesocosm experiment. PLoS One, 2016, 11(12): e0168494. DOI:10.1371/journal.pone.0168494 |
| [21] |
Coughlan J. The estimation of filtering rate from the clearance of suspensions. Marine Biology, 1969, 2(4): 356-358. DOI:10.1007/BF00355716 |
| [22] |
Karatayev AY, Padilla DK, Minchin D et al. Changes in global economies and trade: the potential spread of exotic freshwater bivalves. Biological Invasions, 2007, 9(2): 161-180. DOI:10.1007/s10530-006-9013-9 |
| [23] |
Vaughn CC, Hakenkamp CC. The functional role of burrowing bivalves in freshwater ecosystems. Freshwater Biology, 2001, 46(11): 1431-1446. DOI:10.1046/j.1365-2427.2001.00771.x |
| [24] |
Riisgård HU, Kittner C, Seerup DF. Regulation of opening state and filtration rate in filter-feeding bivalves (Cardium edule, Mytilus edulis, Mya arenaria) in response to low algal concentration. Journal of Experimental Marine Biology and Ecology, 2003, 284(1/2): 105-127. |
| [25] |
Aldridge DW, Payne BS, Miller AC. Oxygen consumption, nitrogenous excretion, and filtration rates of Dreissena polymorpha at acclimation temperatures between 20 and 32℃. Canadian Journal of Fisheries and Aquatic Sciences, 1995, 52(8): 1761-1767. DOI:10.1139/f95-768 |
| [26] |
Diggins TP. Aseasonal comparison of suspended sediment filtration by Quagga (Dreissena bugensis) and Zebra (D. polymorpha) Mussels. Journal of Great Lakes Research, 2001, 27(4): 457-466. DOI:10.1016/S0380-1330(01)70660-0 |
| [27] |
Jørgensen CB, Larsen PS, Riisgård HU. Effect of temperature on the mussel pump. Marine Ecology Progress Series, 1990, 64(1/2): 89-97. |
 2018, Vol. 30
2018, Vol. 30 


