城市水体富营养化修复是当今世界水污染防治的重要组成部分[1].磷是水体富营养化的主要限制因子之一,控制水体磷浓度能有效抑制水体富营养化[2].在外源磷得到有效控制条件下,内源(底泥)磷是水体富营养化的主要贡献者.原位覆盖法[3]能有效控制底泥磷释放,是国内外学者研究热点.污染底泥覆盖材料是原位覆盖技术核心,因此,亟需研发货源广泛、经济有效、可规模化生产的底泥覆盖除磷材料.目前底泥覆盖除磷材料主要分为4大类:铁盐、钙盐、镧改性和铝盐[1].铁盐除磷材料主要有BephosTM[4],但铁磷在还原条件下容易再释放,造成二次污染.钙盐除磷材料主要有NCAP[5],但钙磷在低pH或酸性条件下也易再释放.镧改性的除磷材料主要有phoslock®[6-8]、镧改性磁铁矿[9]和镧改性净水厂污泥[10]等,其中phoslock®在欧美国家得到广泛工程应用[6-8],但其形成的LaPO4非常稳定,能够永久除磷[1],长时间大规模使用镧系除磷材料可能会影响磷的自然循环,甚至导致全球可用磷资源的枯竭.铝盐除磷材料具有较好的磷吸附性能并且吸附的磷不易再释放,是具有较好应用前景的除磷材料[1].
净水厂污泥(WTPS)是净水厂在饮用水处理过程中加入铁盐、铝盐或铁铝盐絮凝沉淀水中悬浮物产生的安全副产物[11-12].相关研究表明,非晶型结构的Fe和Al赋予了WTPS对较多污染物具有高吸附能力,例如磷(P)[13-14]、重金属[15]、有机污染物[16]和硫化氢等[17],其中WTPS对磷的吸附研究深受研究者青睐. WTPS具有高效稳定的磷吸附能力[18-20],并且吸附的磷比较稳定且不易再释放[21].但课题组前期研究表明,WTPS直接使用过程中会向水体中释放氨氮和有机物[22],造成二次污染,因此,课题组自主研发了煅烧改性净水厂污泥(C-WTPS),不仅消除了向水体释放氨氮和有机物问题,而且还增加了磷吸附量[23].
本文以自主研发的C-WTPS作为污染底泥活性覆盖材料,室内静态模拟实验研究C-WTPS覆盖强度对控制底泥磷释放效果的影响,分析C-WTPS和底泥中不同形态磷含量变化,探讨C-WTPS薄层覆盖对上覆水体中pH、溶解氧和氧化还原电位的影响.研究成果可为C-WTPS覆盖控制底泥磷释放的实际应用提供理论依据和技术支撑.
1 材料与方法 1.1 实验材料实验使用的底泥取自华侨大学厦门校区白鹭湖表层底泥.将所取表层底泥通过16目(孔径1 mm)的筛网、混合均匀得到实验底泥,其物理化学性质如表 1所示.实验所用的水为实验室自来水.
| 表 1 底泥物理化学性质 Tab. 1 Physical and chemical properties of sediments |
实验所用的C-WTPS:WTPS取自于厦门某净水厂的脱水后污泥,水厂絮凝剂以铝盐絮凝剂为主,采集的样品质地松软,含水率约为70 % ~75 %,自然风干后样品含水率降至10 % ~13 %.将自然风干的样品放入马弗炉中,在400℃下恒温煅烧2.5 h制得C-WTPS,通过机械破碎筛分获得1~2 mm的颗粒材料,C-WTPS制备参数选择详见参考文献[23],煅烧改性前后污泥理化性质变化详见参考文献[24].
1.2 实验装置与方法实验在容积为5 L、直径150 mm广口玻璃瓶中进行,共有12个广口瓶,分为6组,每组2个平行样,编号为0#~5#,其中,0#为对照系统(未覆盖);1#~5#为C-WTPS覆盖系统,C-WTPS覆盖强度分别为0.25、0.50、1.00、1.50和2.00 kg/m2.每个瓶中底泥重量为1.21 kg,厚度约为5 cm.通过塑料软管利用虹吸原理将自来水缓缓沿瓶壁加入瓶中,上覆水体积为4 L,高度为20 cm.
实验于2018年3月7日开始,4月16日结束,历时40天;玻璃瓶口敞开(不控制上覆水溶解氧(DO)浓度),放置在室内,实验在室温下进行;每天定时测定系统中水深10 cm处水温、pH、DO和氧化还原电位(ORP);定期取50 ml水样,分别测定水样中的总磷(TP)、正磷酸盐,取水样后用自来水进行补充至原刻度线.
实验结束后收集各个系统底泥和C-WTPS.将收到到底泥混合均匀放入冰箱急冻2~3 h,再将其放入冷冻干燥机干燥,再碾细和过100目尼龙筛后待分析;将收集的C-WTPS先用自来水清洗3次,挑选出明显杂质,再用超纯水清洗3次,放入105℃的电烘箱干燥6 h,烘干的C-WTPS再碾细和过100目尼龙筛后待分析.
1.3 项目测试方法 1.3.1 水体相关指标测定方法水温和DO采用HQ30d型便携式溶解氧测定仪测定(HACH);pH采用STARTER3100型便携式pH计(上海奥豪斯仪器有限公司)测定;ORP采用SX712型便携式单参数ORP计(上海三信仪器有限公司)测定;PO43--P和TP浓度采用钼锑抗分光光度法[25]测定.
1.3.2 底泥相关项目测定方法采用SMT法[26-27]测定底泥中不同形态磷的含量,其中包括TP、IP(无机磷)、OP(有机磷)、NAIP(以铁铝磷为主)和AP(以钙磷为主).
1.4 数据处理上覆底泥污染物释放强度计算公式为:
| ${R_n} = \left[ {V\left( {{C_n} - {C_0}} \right) + \sum\limits_{i = 1}^n {{V_i}} \left( {{C_i} - {C_0}} \right)} \right]/(S \cdot t) $ | (1) |
式中,n为取样总次数;Rn为第n次取样时底泥污染物释放强度(mg/(m2·d));V为广口玻璃瓶中上覆水体积(L);Cn为第n次取样时上覆水中污染物浓度(mg/L);C0为初始污染物浓度(mg/L);i为取样次数;Vi为第i次取水样量(L);Ci为第i次取样时污染物浓度(mg/L);S为广口玻璃瓶中底泥-水界面面积(m2);t为污染物释放时间(d).
上覆水磷削减率(P)计算公式为:
| $P = \left( {{C_{{\rm{NCi}}}} - {C_{{\rm{Ci}}}}} \right)/{C_{{\rm{NCi}}}} \times 100\% $ | (2) |
式中,CCi为取样时覆盖系统上覆水磷浓度(mg/L), CNCi为取样时空白系统上覆水磷浓度(mg/L), i为取样次数.
采用Origin 8.5软件通过方差分析研究覆盖系统与空白系统之间削磷污染物效果的差异.
2 结果与讨论 2.1 不同覆盖强度的C-WTPS控制底泥磷释放效果在历时40 d静态模拟实验中,各个系统上覆水温度为18.1~26.3℃;各系统中上覆水中PO43--P和TP浓度变化见图 1.在对照系统上覆水中,PO43--P占TP的74.82 % ~99.15 %,均值为94.42 %,表明白鹭湖底泥释放的磷以正磷酸盐为主;底泥TP的释放强度为6.25~10.87 mg/(m2 ·d),均值为8.90 mg/(m2 ·d).与对照相比,覆盖强度为0.25、0.50、1.00、1.50和2.00 kg/m2的C-WTPS对PO43--P平均削减率分别为60.57 %、76.91 %、90.89 %、94.17 %和97.51 %,对TP平均削减率分别为59.68 %、75.71 %、88.75 %、92.42 %和96.28 %;方差分析表明,覆盖强度为0.25 kg/m2的C-WTPS覆盖系统与对照系统之间削减TP效果无明显差异(P>0.05),其他覆盖强度系统与对照系统均有显著性差异(P<0.05);可见覆盖强度为1 kg/m2以上的C-WTPS能控制底泥中90 %以上TP释放,主要是因为C-WTPS含有较丰富的铁铝非晶结构在材料中形成的活性吸附位点通过配体交换、表面沉淀或络合和微孔扩散等[28-31]方式将磷固定在材料中,从而达到除磷目的(图 1).
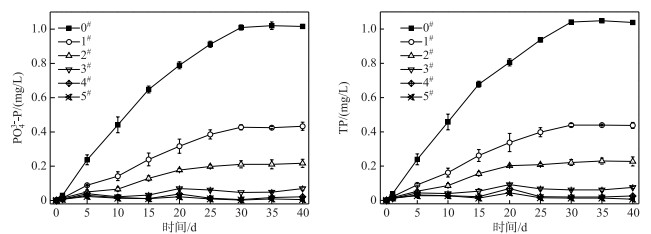
|
图 1 各系统上覆水中PO43--P和TP浓度的变化 Fig.1 Changes of PO43--P and TP concentrations of overlying water in each system |
C-WTPS覆盖系统TP削减率与C-WTPS覆盖强度之间的关系如图 2所示,得到拟合关系式为:y=-15.616x2+53.9887x+49.4992(R2=0.9415),覆盖强度1.73 kg/m2的C-WTPS覆盖系统TP削减率最大理论值为96.20 %,与覆盖强度2.00 kg/m2的TP削减率实验值相近.
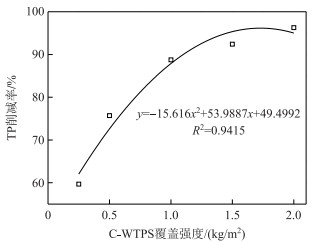
|
图 2 C-WTPS覆盖强度与TP削减率的关系 Fig.2 Relationship between TP reduction rate and C-WTPS capping strength |
在历时40 d静态模拟实验中,各覆盖系统C-WTPS中不同形态磷含量变化见图 3.实验后,C-WTPS中TP、IP和NAIP含量显著性增加(P < 0.05);随着C-WTPS覆盖强度的增加,C-WTPS中TP、IP和NAIP含量均减小;主要原因底泥磷释放强度一定的情况下,C-WTPS覆盖强度越大,材料提供的磷吸附位点越多,可能会出现吸附位点未被充分利用,致使C-WTPS磷吸附量随着覆盖强度的增大而减小.这与课题组前期研究结果一致[32].实验后,各覆盖系统C-WTPS中的TP平均含量分别增加了524.97、464.39、383.63、343.25和242.29 mg/kg;无机磷(IP)平均含量分别增加了504.78、444.20、363.44、323.06和228.10 mg/kg;有机磷(OP)平均含量增加不明显;铁铝结合态磷(NAIP)平均含量分别增加了494.68、424.01、353.34、310.94和204.94 mg/kg;钙磷(AP)平均含量分别增加了16.15、20.19、18.17、8.08和4.04 mg/kg;C-WTPS吸附的磷主要以IP中的NAIP形式存在,OP和AP形式较少[33],与课题组前期研究结果一致[23-24].各覆盖系统中C-WTPS磷吸附量分别为0.53、0.46、0.38、0.34和0.24 mg/g;磷初始浓度为2 mg/L时,C-WTPS的最大吸附量为2.95 mg/g[32],C-WTPS利用效率较低,主要是由于覆盖系统上覆水体中磷浓度较低(均小于0.5 mg/L),致使C-WTPS磷吸附量较低,可见C-WTPS在实际使用时,需要结合实际水体磷初试浓度和C-WTPS覆盖强度估算C-WTPS磷削减量,以免高估C-WTPS除磷效果,致使难达到预期目的.
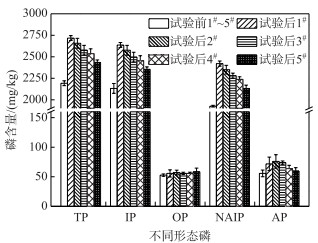
|
图 3 各覆盖系统C-WTPS中不同形态磷含量的变化 Fig.3 Changes of phosphorus content in different forms of C-WTPS in each system |
实验前,底泥中TP、IP、OP、NAIP和AP平均含量分别为3075.13、2832.84、276.63、2187.92和549.20 mg/kg,其中TP中的IP和NAIP分别占92.12 %和71.15 %,说明白鹭湖底泥中磷主要以NAIP为主要无机磷形态存在,底泥释放磷的风险较大.实验后,对照系统底泥中TP、IP、OP、NAIP和AP平均含量分别减少了111.05、100.95、20.19、84.80和-25.24 mg/kg.实验后,各覆盖系统底泥TP含量分别减少了111.05、151.43、181.72、222.10和282.68 mg/kg,分别占试验前底泥TP的3.61 %、4.92 %、5.91 %、7.22 %和9.20 %,底泥磷的迁移转化量为111.05~282.68 mg/kg,底泥磷的迁移转化量与C-WTPS覆盖强度呈正相关(图 4).说明C-WTPS促进了底泥中易释放形态磷迁移到C-WTPS中,并转化较为稳定的形态磷,可见C-WTP覆盖不仅控制了底泥磷释放,而且也削减了底泥磷释放风险.

|
图 4 各系统底泥中不同形态磷含量的变化 Fig.4 Changes of phosphorus content in different forms of sediment in each system |
在历时40 d静态模拟实验中,各系统上覆水中pH的变化如图 5所示. C-WTPS覆盖系统中前15 d期间pH呈现下降趋势,其主要原因是由于浓度差的关系,底泥向上覆水释放各类阴阳离子(如金属离子、氮磷营养盐等);上覆水从好氧向缺氧状态转变,ORP的降低等因素改变了上覆水中铁盐、硫酸盐等的种类和浓度,导致上覆水pH的下降[24, 34-36].实验15 d后,方差分析表明,对照系统和各覆盖系统的pH均存在显著性差异(P < 0.05),并且C-WTPS覆盖强度越大pH差异越显著.说明C-WTPS能够改变水体pH,且当上覆水显碱性时,pH会下降;当上覆水显酸性时,pH会上升,但始终在pH=7小范围内波动.这与课题组前期研究结果一致[24].其原因有,首先是C-WTPS中含有大量的无定型铝铁化合物,C-WTPS吸附磷的方式为表面络合、配体(离子)交换、表面沉淀等方式可能改变了上覆水阴阳离子(如H+和金属离子)的种类和浓度. Yang等[31]研究表明C-WTPS在配体(离子)交换过程会释放H+、TOC、金属离子和其他阴离子,释放的H+和金属离子水解产生的H+导致了上覆水pH的减小;其次是上覆水溶解氧小于1 mg/L处于缺氧状态下,微生物分解有机物可能产生有机酸,导致pH下降[36].
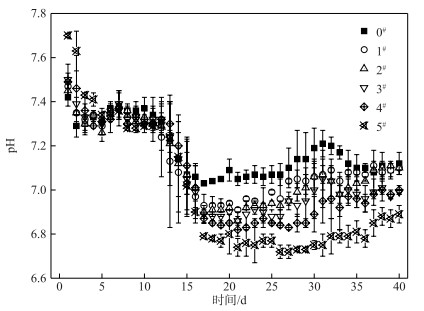
|
图 5 各系统上覆水中pH变化 Fig.5 Changes of pH of overlying water in each system |
在历时40 d静态模拟实验中,各系统上覆水中DO和ORP的变化如图 6所示.
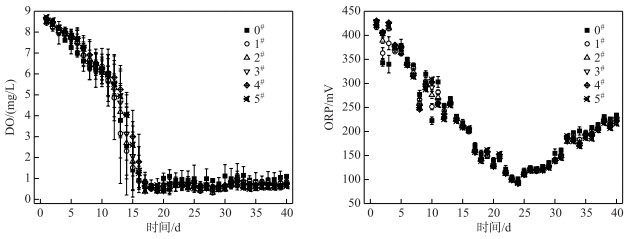
|
图 6 各系统上覆水中DO浓度和ORP的变化 Fig.6 Changes of DO concentrations and ORP of overlying water in each system |
各系统上覆水体中的DO浓度均呈现先降低后保持平稳的趋势;在前15 d各系统从好氧状态逐渐变成缺氧状态,实验15 d后各系统均为缺氧状态(DO<1 mg/L),方差分析表明,C-WTPS覆盖系统上覆水中DO浓度与对照系统没有显著性差异(P>0.05);可见各系统耗氧速率大于空气复氧速率,说明C-WTPS覆盖控制底泥有机物释放效果不明显,从而改善上覆水体DO环境的效果不明显(图 6).
各系统上覆水中的ORP均呈现先降低后增加的趋势(图 6).在24 d时达到最小值为97 mV,其原因可能是DO和pH降低逐渐使还原态与氧化态物质浓度比值增大导致ORP减小;在24 d后DO浓度趋于稳定,系统中的还原态物质开始逐渐减少导致ORP增加;此外还有离子浓度和温度等因素也会间接影响ORP[37].方差分析表明,C-WTPS覆盖系统上覆水中ORP与对照系统没有显著性差异(P>0.05),说明C-WTPS覆盖改善上覆水中ORP环境的效果不明显.
3 结论1) 覆盖强度为0.25、0.50、1.00、1.50和2.00 kg/m2的C-WTPS对PO43--P平均削减率分别为60.57 %、76.91 %、90.89 %、94.17 %和97.51 %,对TP平均削减率分别为59.68 %、75.71 %、88.75 %、92.42 %和96.28 %;可见覆盖强度为1.00 kg/m2以上的C-WTPS能控制底泥中90 %以上TP的释放.
2) C-WTPS吸附的磷主要以无机磷中的铁铝结合态磷形式存在,C-WTPS促进了底泥中易释放形态磷迁移到C-WTPS中,并转化较为稳定的形态磷.
3) C-WTPS覆盖后,上覆水体中pH开始呈现下降趋势,最终维持在pH=7范围波动;C-WTPS覆盖改善上覆水体中DO和ORP环境的效果不明显.
| [1] |
Lürling M, Mackay E, Reitzel K et al. Editorial-A critical perspective on geo-engineering for eutrophication management in lakes. Water Research, 2016, 97: 1-10. DOI:10.1016/j.watres.2016.03.035 |
| [2] |
Lewis WM, Wurtsbaugh WA, Paerl HW. Rationale for control of anthropogenic nitrogen and phosphorus to reduce eutrophication of inland waters. Environmental Science&Technology, 2011, 45(24): 10300-10305. |
| [3] |
Spears BM, Maberly SC, Pan G et al. Geo-engineering in lakes:a crisis of confidence?. Environmental Science&Technology, 2014, 48: 9977-9979. |
| [4] |
Zamparas M, Drosos M, Georgiou Y et al. A novel bentonite-humic acid composite material BephosTM for removal of phosphate and ammonium from eutrophic waters. Chemical Engineering Journal, 2013, 225: 43-51. DOI:10.1016/j.cej.2013.03.064 |
| [5] |
Ding SM, Chen MS, Cui JZ et al. Reactivation of phosphorus in sediments after calcium-rich mineral capping:Implication for revising the laboratory testing scheme for immobilization efficiency. Chemical Engineering Journal, 2018, 331: 720-728. DOI:10.1016/j.cej.2017.09.010 |
| [6] |
Douglas GB, Lurling M, Spears BM. Assessment of changes in potential nutrient limitation in an impounded river after application of lanthanum-modified bentonite. Water Research, 2016, 97: 47-54. DOI:10.1016/j.watres.2016.02.005 |
| [7] |
Waajen G, Van OF, Douglas G et al. Management of eutrophication in Lake De Kuil (The Netherlands) using combined flocculant-Lanthanum modified bentonite treatment. Water Research, 2016, 97: 83-95. DOI:10.1016/j.watres.2015.11.034 |
| [8] |
Ding SM, Sun Q, Chen X et al. Synergistic adsorption of phosphorus by iron in lanthanum modified bentonite (Phoslock):New insight into sediment phosphorus immobilization. Water Research, 2018, 134: 32-43. DOI:10.1016/j.watres.2018.01.055 |
| [9] |
Fang L, Liu R, Li J et al. Magnetite/Lanthanum hydroxide for phosphate sequestration and recovery from lake and the attenuation effects of sediment particles. Water Research, 2018, 130: 243-254. DOI:10.1016/j.watres.2017.12.008 |
| [10] |
Wang C, Wu Y, Wang Y et al. Lanthanum-modified drinking water treatment residue for initial rapid and long-term equilibrium phosphorus immobilization to control eutrophication. Water Research, 2018, 137: 173-183. DOI:10.1016/j.watres.2018.03.018 |
| [11] |
Ippolito JA, Barbarick KA, Elliott HA. Drinking water treatment residuals:a review of recent uses. Journal of Environmental Quality, 2011, 40: 1-12. DOI:10.2134/jeq2010.0242 |
| [12] |
Okuda T, Nishijima W, Sugimoto M et al. Removal of coagulant aluminum from water treatment residuals by acid. Water Research, 2014, 60: 75-81. DOI:10.1016/j.watres.2014.04.041 |
| [13] |
Li Z, Jiang N, Wu F et al. Experimental investigation of phosphorus adsorption capacity of the waterworks sludges from five cities in China. Ecological Engineering, 2013, 53: 165-172. DOI:10.1016/j.ecoleng.2012.12.038 |
| [14] |
Wang C, Liang J, Pei Y et al. A method for determining the treatment dosage of drinking water treatment residuals for effective phosphorus immobilization in sediments. Ecological Engineering, 2013, 60(11): 421-427. |
| [15] |
Lin L, Xu X, Papelis C et al. Innovative use of drinking water treatment solids for heavy metals removal from desalination concentrate:synergistic effect of salts and natural organic matter. Chemical Engineering Research&Design, 2017, 120: 231-239. |
| [16] |
Punamiya P, Sarkar D, Rakshit S et al. Effectiveness of aluminum-based drinking water treatment residuals as a novel sorbent to remove tetracyclines from aqueous medium. Journal of Environmental Quality, 2013, 42: 1449-1459. DOI:10.2134/jeq2013.03.0082 |
| [17] |
Wang CH, Pei YS. The removal of hydrogen sulfide in solution by ferric and alum water treatment residuals. Chemosphere, 2012, 88: 1178-1183. DOI:10.1016/j.chemosphere.2012.03.065 |
| [18] |
Agyin-Birikorang S, O'Connor GA. Lability of drinking water treatment residuals (WTR) immobilized phosphorus:aging and pH effects. Journal of Environmental Quality, 2007, 36: 1076-1085. DOI:10.2134/jeq2006.0535 |
| [19] |
Oliver IW, Grant CD, Murray RS. Assessing effects of aerobic and anaerobic conditions on phosphorus sorption and retention capacity of water treatment residuals. Journal of Environmental Management, 2011, 92: 960-966. |
| [20] |
Wang CH, Wang ZY, Lin L et al. Effect of low molecular weight organic acids on phosphorus adsorption by ferric-alum water treatment residuals. Journal of Hazardous Materials, 2012, 203/204: 145-150. DOI:10.1016/j.jhazmat.2011.11.084 |
| [21] |
Wang CH, Fei CB, Pei YS. Stability of P saturated water treatment residuals under different levels of dissolved oxygen. Clean Soil Air Water, 2012, 40: 844-849. DOI:10.1002/clen.v40.8 |
| [22] |
Huang HS, Yang ZM, Zhou ZM et al. Efficiency of controlling nitrogen and phosphorus release from sediment using thin-layer capping with water treatment plant sludge. Journal of Huaqiao University:Natural Science, 2016, 37(3): 347-351. [黄华山, 杨志敏, 周真明等. 净水厂污泥覆盖控制底泥氮磷释放效果. 华侨大学学报:自然版, 2016, 37(3): 347-351.] |
| [23] |
Liu QD, Zhou ZM, Zhang HZ et al. Parameter optimization of preparing phosphorus removal material by using calcined water treatment plant sludge. Journal of Huaqiao University:Natural Science, 2018, 39(1): 51-56. [刘啟迪, 周真明, 张红忠等. 煅烧改性净水厂污泥制备除磷材料工艺参数优化. 华侨大学学报:自然科学版, 2018, 39(1): 51-56.] |
| [24] |
Zhou Z, Liu Q, Li S et al. Characterizing the correlation between dephosphorization and solution pH in a calcined water treatment plant sludge. Environmental Science&Pollution Research, 2018, 25(19): 18510-18518. |
| [25] |
Ministry of Environmental Protection of the People's Republic of China, Editorial Board of Water and Wastewater Monitoring and Analysis Methods ed.Water and wastewater monitoring and analysis methods:4th edition.Beijing:China Environmental Science Press, 2002. [国家环境保护总局《水和废水监测分析方法》编委会.水和废水监测分析方法:第4版.北京:中国环境科学出版社, 2002. ]
|
| [26] |
Ruban V, Lópezsánchez JF, Pardo P et al. Selection and evaluation of sequential extraction procedures for the determination of phosphorus forms in lake sediment. Journal of Environmental Monitoring Jem, 1999, 1(1): 51-56. DOI:10.1039/a807778i |
| [27] |
González Medeiros JJ, Pérez CB, Fernández GE. Analytical phosphorus fractionation in sewage sludge and sediment samples. Analytical and Bioanalytical Chemistry, 2005, 381(4): 873-878. DOI:10.1007/s00216-004-2989-z |
| [28] |
Ippolito JA, Barbarick KA, Heil DM et al. Phosphorus retention mechanisms of a water treatment residual. Journal of Environmental Quality, 2003, 32(5): 1857-1864. DOI:10.2134/jeq2003.1857 |
| [29] |
Makris KC.Long-term stability of sorbed phosphorus by drinking-water treatment residuals: mechanisms and implications[Dissertation].Florida: University of Florida, 2004.
|
| [30] |
Al-Tahmazi T, Babatunde AO. Mechanistic study of P retention by dewatered waterworks sludges. Environmental Technology&Innovation, 2016, 6: 38-48. |
| [31] |
Yang Y, Zhao YQ, Babatunde AO et al. Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Separation&Purification Technology, 2006, 51(2): 193-200. |
| [32] |
Liu QD.Study on preparation of water environmental phosphorus removal materials by calcining water treatment plant sludge[Dissertation].Xiamen: Huaqiao University, 2018. [刘啟迪.净水厂污泥煅烧制备水环境除磷材料[学位论文].厦门: 华侨大学, 2018. ]
|
| [33] |
Hou QJ, Meng PP, Pei HY et al. Phosphorus adsorption characteristics of alum sludge:adsorption capacity and the forms of phosphorus retained in alum sludge. Materials Letters, 2018, 229: 31-35. DOI:10.1016/j.matlet.2018.06.102 |
| [34] |
Wu YH, Wen YJ, Zhou JX et al. Phosphorus release from lake sediments:effects of pH, temperature and dissolved oxygen. KSCE Journal of Civil Engineering, 2014, 18(1): 323-329. DOI:10.1007/s12205-014-0192-0 |
| [35] |
Li ZR, Sheng YQ, Yang J et al. Phosphorus release from coastal sediments:Impacts of the oxidation-reduction potential and sulfide. Marine Pollution Bulletin, 2016, 113(1/2): 176-181. |
| [36] |
Huang TL, Zhou RY, Xia C et al. Effects of oxidation-reduction potential and microorganism on the release of phosphorus from sediments. Environmental Chemistry, 2014, 33(6): 930-936. [黄廷林, 周瑞媛, 夏超等. 氧化还原电位及微生物对水库底泥释磷的影响. 环境化学, 2014, 33(6): 930-936.] |
| [37] |
Xiang J, Xu LP, Li HP et al. Research on and application of oxidation-reduction potential. Earth and Environment, 2014, 42(3): 430-436. [向交, 徐丽萍, 李和平等. 氧化还原电位的研究及应用. 地球与环境, 2014, 42(3): 430-436.] |
 2019, Vol. 31
2019, Vol. 31 

