(2: 国家科学与生产部白俄罗斯国家科学院生物能源科学-实践中心, 明斯克 220047)
(3: 康奈尔大学自然资源系, 纽约 14853)
(4: 奥尔胡斯大学生物科学系, 锡尔克堡 8600)
(5: 中东科技大学生物科学学院, 生态系统研究与应用中心/湖沼学实验室, 安卡拉 06800)
(6: 中国丹麦科研教育中心(SDC), 北京 101408)
(7: 暨南大学生态学系/水生生物研究所, 广州 510632)
(8: 中国科学院南京地理与湖泊研究所, 湖泊与环境国家重点实验室, 南京 210008)
(2: State Scientific and Production Amalgamation Scientific-practical Center of the National Academy of Sciences of Belarus for Biological Resources, Minsk 220047, Belarus)
(3: Department of Natural Resources, Cornell University, New York 14853, USA)
(4: Department of Bioscience, Aarhus University, Silkeborg 8600, Denmark)
(5: Limnology Laboratory, Department of Biological Sciences and Centre for Ecosystem Research and Implementation, Middle East Technical University, Ankara 06800, Turkey)
(6: Sino-Danish Centre for Education and Research (SDC), Beijing 101408, P. R. China)
(7: Department of Ecology and Institute of Hydrobiology, Jinan University, Guangzhou 510632, P. R. China)
(8: State Key Laboratory of Lake Science and Environment, Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, P. R. China)
浅水水体具有明显的水层浮游生境和底部底栖生境. 浮游生境中的生物主要营浮游生活,包括细菌、病毒、浮游藻类、浮游动物以及一些营浮游生活的水生昆虫和鱼类. 该生境中的能量流动主要通过浮游藻类和细菌途径[1]. 而底栖生境中的生物主要是底栖藻类、大型水生植物、细菌、水生昆虫、底栖无脊椎动物和一些鱼类等. 通常底栖生境中的能量流动较浮游生境中更为复杂. 浅水水体中的浮游生境和底栖生境间存着强烈的耦合作用[2-4],底栖藻类和浮游藻类对光照和营养盐的竞争是浅水水体底栖浮游生境耦合最为重要的生态过程之一,且该过程受杂食性鱼类的影响. 本文就杂食性鱼类对浅水水体底栖浮游生境耦合作用的影响进行了微综述.
1 浅水水体底栖浮游生境耦合作用浅水水体中底栖浮游生境耦合作用是指底栖生境所发生的生态过程受到浮游生境的影响,相反,浮游生境中所发生的过程也受到底栖生境的影响[1, 5-6]. 浮游生境对底栖生境最显著的作用(向下)包括光照未达底栖生境前被水层截获,即光照在浮游生境中的衰减,可影响底泥表层的底栖植物(沉水植物、底栖藻类等)生长[5-6]. 底栖生境对浮游生境最为显著的作用(向上)包括底栖植物对底泥营养盐,如抑制底泥磷(P)向水层释放,影响水层浮游藻类的生长及浮游生境变化[1, 7]. 因此,底栖浮游生境耦合作用对浅水水体生态系统的结构、功能起到关键作用,对水体富营养化过程产生重要影响,近年来成为研究的热点[8-11].
在缺少大型水生植物的浅水水体中,底栖藻类和浮游藻类对光照和营养盐的竞争是底栖浮游生境耦合最为重要的生态过程之一. 然而,相较于大型水生植物,在浅水水体中这一耦合过程中底栖藻类受到的关注较少. 在一些“清水态”浅水湖泊中,底栖藻类常是主要初级生产者[12-13];即便以大型水生植被为主的浅水系统中也存在着大量底栖藻类. 因此,本文重点以底栖藻类为代表的底栖生境与浮游生境耦合作用进行微综述.
2 底栖藻类和浮游藻类对光照和营养盐的竞争是浅水水体底栖浮游生境耦合最为重要的生态过程之一底栖藻类和浮游藻类可共存于水质较好的浅水水体中,对光照和营养盐存在竞争关系[3, 14]. 浮游藻类生长在水层浮游生境中,而底栖藻类生长在浅水水体底部的底泥表面. 光照常成为底栖藻类生长的限制性因子,浅水水体中底泥表层即便增加少量的光照也利于促进底栖藻类生长,一些底栖藻类甚至可在很弱的光照条件下生长[15]. 如有研究表明,底栖藻类生长的光补偿点可低至4.7 μmol/(m2 ·s)[16]. 然而底栖藻类群落结构中不同种类间存在差异,比如转板藻(Mougeotia)的光照补偿点为10 μmol/(m2 ·s)[17],而刚毛藻(Cladophora glomerata)的光照补偿点为29 μmol/(m2 ·s)[18].
由于生境的不同,底栖和浮游藻类对光照和营养盐的竞争是不对称的[3]. 浮游藻类会优先获得光照,降低到达底栖生境的光照强度. 若光照在水层浮游生境中大量衰减,会制约底栖藻类生长[19],致使底栖藻类生长受限,甚至消失. 底栖藻类最先接触底泥释放的营养盐(内源),能通过物理阻隔和生长过程中引起的化学反应减少底泥中营养盐,特别是磷的释放[7, 20](图 1),从而降低浮游生境中营养盐浓度,这将限制浮游藻类的生长[19]. 因此,在水质较好的浅水系统中浮游藻类对光照具有竞争优势,而底栖藻类对营养盐具有竞争优势. 但随着浮游生境中营养盐浓度的上升,浮游藻类可获得充足的营养盐而大量生长,增加光在水层中的衰减,降低到达底泥表面的光照,限制底栖藻类的生长[3, 21],底栖藻类逐步失去对营养盐的竞争优势. 底栖藻类的减少,甚至消失则有利于底泥营养盐的释放,进一步促进浮游藻类生长,加剧水体富营养化[7, 20].
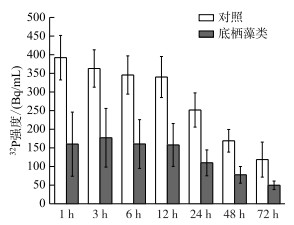
|
图 1 底栖藻类对底泥磷释放的影响(改自文献[20])(实验起始时,148 kBq NaH232PO4通过实验装置的注射孔被分别注射入无底栖藻类的对照组和具有底栖藻类处理的底泥,之后分别采样并测定上覆水中32P的强度) Fig.1 Effects of benthic algae on sediment phosphorus release (Revised from reference [20]) (At time zero, 148 kBq NaH232PO4 were added through the injection hole into each sediment core, in both controls and experimental tubes. The sediment surface was covered by benthic algae in the experimental tubes, while non-algae containing tubes served as controls) |
浅水水体中底栖藻类和浮游藻类对光照和营养盐的竞争结果常呈现此消彼长的动态变化[2-3](底栖藻类生产占全湖初级生产比例与水中磷浓度的关系见图 2). 该动态变化不仅会导致初级生产者改变(底栖藻类占优势还是浮游藻类占优势),还影响着水生态系统的结构和功能[19, 21],决定着浅水生态系统的关键特征[9]. 在这一竞争耦合过程中,若浮游藻类占据优势,则水质恶化,水体浑浊,生态系统呈“浊水态”,浮游藻类成为主要初级生产者并可成为鱼类饵料的主要来源[22-23],如在一些富营养化湖泊中浮游藻类可占整个湖泊初级生产力的95 % 以上[24]. 而当底栖藻类成为优势时,水体常清澈,水质较好,生态系统呈“清水态”,底栖藻类成为主要初级生产者,如在一些清澈的浅水湖泊中,底栖藻类可占整个湖泊初级生产力的95 % 以上[2]. 因此,底栖藻类与浮游藻类对光照和营养盐竞争的结果是决定生态系统特征的关键因素之一,成为浅水生态系统研究的热点之一[3, 10, 19].
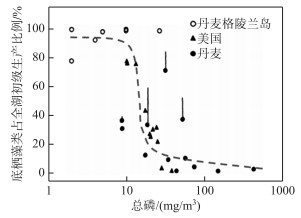
|
图 2 底栖藻类生产占全湖初级生产比例与水中总磷浓度的关系(改自文献[2]) Fig.2 The relative contribution of benthic algae to whole lake primary production as a function of total phosphorus concentration (Revised from reference[2]) |
影响底栖浮游生境耦合作用的因素很多,如底质类型、气候状况、水动力条件、鱼类、底栖动物、水生植物等均对底栖水层生境耦合作用产生重要影响. 本文以杂食性鱼类为重点进行介绍.
4.1 杂食性鱼类的影响概述鱼类作为水生系统中的重要消费者,不仅可通过“下行效应”对初级生产产生重要影响,而且也能通过扰动底泥和排泄营养盐等途径影响水中营养盐含量及形态,从而通过“上行效应”影响初级生产力的分布,对底栖浮游生境耦合作用的影响备受关注[6, 25-27].
广义上,大多数鱼类均为杂食性,然而根据鱼类的主要食物组成,仍可将鱼类分成草食性、肉食性、杂食性鱼类等. 杂食性鱼类分布广[28-31],因兼具肉食性、植食性和滤食性鱼类的特点,对水生态环境产生的影响深远、复杂. 因此,理清杂食性鱼类对底栖浮游生境耦合作用的影响,是淡水生态系统有效管理的关键[32-33].
在围隔或实验室实验中发现,杂食性鱼类可滤食水中的浮游藻类,对浮游藻类产生较大的摄食压力,能有效降低浮游藻类数量[34-36];在水库研究中也发现杂食性鱼类可直接滤食浮游藻类[37-38];在蓝藻大量生长的重度富营化水体中也发现,杂食性鱼类能有效控制浮游藻类大量生长、提高水体透明度[35, 39-40];但杂食性鱼类虽可滤食大粒径浮游藻类,却无法有效去除小粒径浮游藻类[37-38]. 除此之外,杂食性鱼类对水体氮、磷等营养盐浓度及形态的影响存在着较大的不确定性[35, 39-40].
浅水系统中杂食性鱼类除滤食浮游藻类、浮游动物、悬浮颗粒物外,还可牧食底栖生物,包括底栖藻类[41];杂食性鱼类也可促进底泥再悬浮、增加底泥磷释放[35, 42];杂食性鱼类也通过排泄释放磷,升高水层中营养盐浓度[43-44](图 3). 然而,不同的杂食性鱼类(如底栖杂食性鱼类、偏植物性饵料的杂食性鱼类、偏动物性饵料的杂食性鱼类以及小型杂食性鱼类)对底栖浮游生境耦合的影响机理不同,产生的生态环境效应各异.
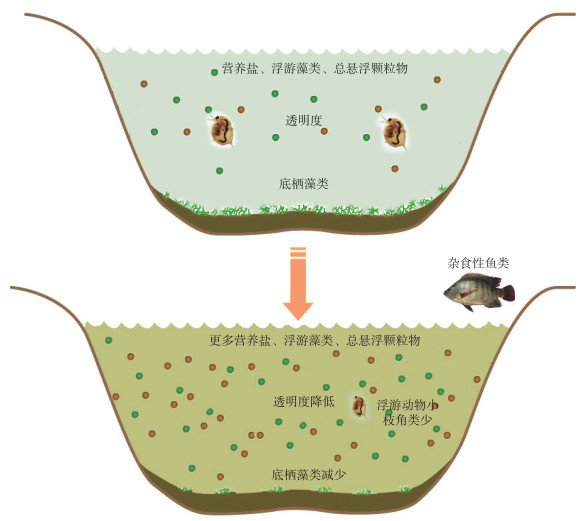
|
图 3 杂食性鱼类对底栖浮游生境耦合的影响示意图 Fig.3 Illustration of the effects of omnivorous fish on benthic-pelagic habitats coupling |
底栖杂食性鱼类,如鲫(Carassius auratus)和鲤(Cyprinus carpio),可直接摄食底栖生物[45],包括底栖动物、水生昆虫、水生植物和底栖藻类等. 底栖杂食性鱼类,特别是鲤在摄食过程中会搅动底泥,加剧水体底泥再悬浮. 研究表明有些底栖杂食性鱼类,如鲤在底栖生境摄食时可深达底泥的12 cm处,产生强烈的生物扰动效应,可影响到底泥的深处[46]. 有研究表明底栖食性鱼类的密度与水体中悬浮颗粒物浓度呈显著正相关关系,从而降低水体透明度,抑制底栖藻类生长;底栖杂食性鱼类能显著促进底泥营养盐释放,增加水层中营养盐浓度[27, 47],从而促进浮游藻类生长. 另外,浅水水体中底栖杂食性鱼类,如鲤鱼,也可减少浮游动物,改变浮游动物的群落结构. 因此,底栖杂食性鱼类不利于底栖藻类生长,从而促进浮游藻类的生长,加剧水体富营养化程度.
4.3 偏植物性饵料的杂食性鱼类偏植物性饵料的杂食性鱼类,如尼罗罗非鱼(Oreochromis niloticus),其食物包括浮游藻类、浮游动物、水生植物的叶子、底栖生物、水生无脊椎动物、有机碎屑等. 研究表明,罗非鱼可显著促进底泥磷的释放,增加水体浑浊度和营养盐浓度,促进浮游藻类生长,恶化水下光照条件,抑制底栖藻类竞争. 即罗非鱼可显著增加水中的总氮、总磷浓度及浮游藻类密度,提高总悬浮颗粒物浓度,降低底泥表层光照,引起底栖藻类生物量下降[33];同时罗非鱼虽为杂食性鱼类,其食性会随发育阶段而有所不同,幼鱼时期主要摄食浮游生物,以摄食轮虫为主,在幼鱼期后,消化器官已发育完善,能够捕食枝角类等浮游动物,在随后的生长中逐渐发展了滤食浮游藻类和碎屑的功能,能够大量滤食蓝藻、硅藻等浮游藻类. 罗非鱼摄食时可用鳃分泌粘液包裹住浮游生物细胞,因而可以摄食小于5 μm的微型浮游藻类[39]. 但也有研究表明,根据其滤食器官鳃耙的结构,也可滤食直径大于50 μm的浮游藻类和浮游动物[34, 37],特别是对枝角类的摄食使浮游动物对浮游藻类压力减小,利于小粒径浮游藻类生长,从而改变浮游植物群落结构. 因此,偏植物性饵料的杂食性鱼类不利于透明度的提高与水质的改善.
4.4 偏动物性饵料的杂食性鱼类一般认为食鱼性鱼类可捕食食浮游生物的鱼类,从而减轻浮动动物的生存压力,利于扩大浮游动物种群,从而增强浮游动物对浮游藻类的牧食压力以遏制浮游藻类生长,进而改善水质.
偏动物性饵料的杂食性鱼类,如中华沙鳅(Botia superciliaris)、黄鳝(Monopterus albus)等为杂食性鱼类,偏动物性饵料,主要摄食水生昆虫幼虫、甲壳类、底栖无脊椎动物、浮游生物、小鱼等. 在自然水域中,处于不同生长发育阶段的偏动物性饵料的杂食性鱼类,其食物组成也不尽相同. 如鳝苗阶段,其食物以原生动物、轮虫、枝角类、桡足类等小型浮游动物为主,也摄食一些浮游藻类,如硅藻、黄藻、绿藻、裸藻等;幼鳝与成鳝阶段,其生活方式逐渐转为底栖性,因此食物也逐渐转变为各种水陆生蠕虫、昆虫及其幼虫,如水丝蚓(红虫)、摇蚊幼虫以及各种蜻蜓幼虫或动物卵,甚至小鱼等. 而关于偏动物性饵料的杂食性鱼类,是有利于水质改善,还是有利于浮游藻类生长,仍需深入研究.
4.5 小型杂食性鱼类小型杂食性鱼类,如餐条(Hemicculter leuciclus)、麦穗鱼(Pseudorashora parva)、鳑鲏亚科的种类,主要以高等水生植物的叶片、碎屑和藻类为食,也可摄食浮游动物、底栖无脊椎动物如水生昆虫等. 研究表明,尽管大鳍鱊(Acheilognathus macropterus) 不像个体较大的鲫、鲤或罗非鱼等杂食性鱼类对底泥扰动强烈,但仍会对底栖浮游生境产生影响,主要通过排泄营养的途径提高水层氮、磷浓度,促进了浮游藻类生长[48];同时小型杂食性鱼类,如鳑鲏亚科的种类,可显著增加轮虫的数量,但会降低枝角类密度[48]. 因此,小型杂食性鱼类可能主要通过增加浮游藻类生物量的途径,影响浅水水体的底栖浮游生境耦合过程.
5 小结与展望浅水水体存在着强烈的底栖浮游生境耦合作用,在一些“清水态”浅水湖泊中,底栖藻类通常是主要初级生产者;即便以大型水生植被为主的浅水系统中也存在着大量底栖藻类. 底栖藻类和浮游藻类对光照和营养盐的竞争是该生境耦合最为重要的生态过程之一,该生境耦合的结果决定水生态系统关键特征. 杂食性鱼类对该耦合作用产生重要影响,总体而言,杂食性鱼类不利于底栖藻类生长,有利于浮游藻类竞争,从而加重水体富营养化而恶化水质. 但不同种类的杂食性鱼类仍因食性的差异对底栖浮游生境耦合的影响机理不同,产生的生态环境效应各异;即便同一种杂食性鱼类也可因发育阶段不同,对底栖浮游生境耦合产生不同的影响.
杂食性鱼类因食性可塑性强、分布广[28-31],兼具肉食性和植食性鱼类的特点,对水生态环境产生的影响深远、复杂;此外,鱼类群落结构在人类活动、全球变暖以及富营养化等多重因子胁迫下发生改变[49],杂食性鱼类比例上升[50-51]. 因此,杂食性鱼类对水生态环境产生的影响可能随之增加,值得持续关注.
| [1] |
Schindler DE, Scheuerell MD. Habitat coupling in lake ecosystems. Oikos, 2002, 98(2): 177-189. DOI:10.1034/j.1600-0706.2002.980201.x |
| [2] |
Vadeboncoeur Y, Jeppesen E, Zanden MJV et al. From Greenland to green lakes: Cultural eutrophication and the loss of benthic pathways in lakes. Limnology and Oceanography, 2003, 48(4): 1408-1418. DOI:10.4319/lo.2003.48.4.1408 |
| [3] |
Jäger CG, Diehl S. Resource competition across habitat boundaries: Asymmetric interactions between benthic and pelagic producers. Ecological Monographs, 2014, 84(2): 287-302. DOI:10.1890/13-0613.1 |
| [4] |
Blottière L, Jaffar-Bandjee M, Jacquet S et al. Effects of mixing on the pelagic food web in shallow lakes. Freshwater Biology, 2017, 62(1): 161-177. DOI:10.1111/fwb.12859 |
| [5] |
Marcus NH, Boero F. Minireview: The importance of benthic-pelagic coupling and the forgotten role of life cycles in coastal aquatic systems. Limnology and Oceanography, 1998, 43(5): 763-768. DOI:10.4319/lo.1998.43.5.0763 |
| [6] |
Soetaert K, Middelburg JJ, Herman PMJ et al. On the coupling of benthic and pelagic biogeochemical models. Earth-Science Reviews, 2000, 51(1/2/3/4): 173-201. DOI:10.1016/S0012-8252(00)00004-0 |
| [7] |
Spears BM, Carvalho L, Perkins R et al. Effects of light on sediment nutrient flux and water column nutrient stoichiometry in a shallow lake. Water Research, 2008, 42(4/5): 977-986. DOI:10.1016/j.watres.2007.09.012 |
| [8] |
Alonso-Pérez F, Ysebaert T, Castro CG. Effects of suspended mussel culture on benthic-pelagic coupling in a coastal upwelling system (Ría de Vigo, NW Iberian Peninsula). Journal of Experimental Marine Biology and Ecology, 2010, 382(2): 96-107. DOI:10.1016/j.jembe.2009.11.008 |
| [9] |
Genkai-Kato M, Vadeboncoeur Y, Liboriussen L et al. Benthic-planktonic coupling, regime shifts, and whole-lake primary production in shallow lakes. Ecology, 2012, 93(3): 619-631. DOI:10.1890/10-2126.1 |
| [10] |
Zhang XF, Liu ZW, Jeppesen E et al. Effects of deposit-feeding tubificid worms and filter-feeding bivalves on benthic-pelagic coupling: Implications for the restoration of eutrophic shallow lakes. Water Research, 2014, 50: 135-146. DOI:10.1016/j.watres.2013.12.003 |
| [11] |
Liu ZW, Zhang XF, Chen FZ et al. The responses of the benthic-pelagic coupling to eutrophication and regime shifts in shallow lakes: Implication for lake restoration. J Lake Sci, 2020, 32(1): 1-10. [刘正文, 张修峰, 陈非洲等. 浅水湖泊底栖—敞水生境耦合对富营养化的响应与稳态转换机理: 对湖泊修复的启示. 湖泊科学, 2020, 32(1): 1-10. DOI:10.18307/2020.0101] |
| [12] |
Vadeboncoeur Y, Steinman AD. Periphyton function in lake ecosystems. The Scientific World Journal, 2002, 2: 1449-1468. DOI:10.1100/tsw.2002.294 |
| [13] |
Dalsgaard T. Benthic primary production and nutrient cycling in sediments with benthic microalgae and transient accumulation of macroalgae. Limnology and Oceanography, 2003, 48(6): 2138-2150. DOI:10.4319/lo.2003.48.6.2138 |
| [14] |
Yu X, Zhang XF, Liu ZW. The effect of Anodonta woodiana on the competitive relationship between benthic and planktonic algae in shallow aquatic ecosystem. Ecologic Science, 2012, 31(3): 301-305. [喻晓, 张修峰, 刘正文. 背角无齿蚌(Anodonta woodiana)对浅水系统底栖和浮游藻类竞争关系的影响. 生态科学, 2012, 31(3): 301-305. DOI:10.3969/j.issn.1008-8873.2012.03.013] |
| [15] |
Steinman AD, McIntire CD. Effects of irradiance on the community structure and biomass of algal assemblages in laboratory streams. Canadian Journal of Fisheries and Aquatic Sciences, 1987, 44(9): 1640-1648. DOI:10.1139/f87-199 |
| [16] |
Wiencke C ed. Biology of polar benthic algae. Berlin: Walter de Gruyter, 2011.
|
| [17] |
Graham JM, Arancibia-Avila P, Graham LE. Physiological ecology of a species of the filamentous green algaMougeotia under acidic conditions: Light and temperature effects on photosynthesis and respiration. Limnology and Oceanography, 1996, 41(2): 253-262. DOI:10.4319/lo.1996.41.2.0253 |
| [18] |
Lorenz RC, Monaco ME, Herdendorf CE. Minimum light requirements for substrate colonization by Cladophora glomerata. Journal of Great Lakes Research, 1991, 17(4): 536-542. DOI:10.1016/S0380-1330(91)71389-0 |
| [19] |
Pasternak A, Hillebrand H, Flöder S. Competition between benthic and pelagic microalgae for phosphorus and light-long-term experiments using artificial substrates. Aquatic Sciences, 2009, 71(2): 238-249. DOI:10.1007/s00027-009-9143-0 |
| [20] |
Zhang XF, Liu ZW, Gulati RD et al. The effect of benthic algae on phosphorus exchange between sediment and overlying water in shallow lakes: A microcosm study using 32P as tracer. Hydrobiologia, 2013, 710(1): 109-116. DOI:10.1007/s10750-012-1134-9 |
| [21] |
Flöder S, Combüchen A, Pasternak A et al. Competition between pelagic and benthic microalgae for phosphorus and light. Aquatic Sciences, 2006, 68(4): 425-433. DOI:10.1007/s00027-006-0824-7 |
| [22] |
Vadeboncoeur Y, Vander Zanden MJ, Lodge DM. Putting the lake back together: Reintegrating benthic pathways into lake food web models. BioScience, 2002, 52(1): 44. DOI:10.1641/0006-3568(2002)052[0044:ptlbtr]2.0.co;2 |
| [23] |
Vander Zanden MJ, Chandra S, Park SK et al. Efficiencies of benthic and pelagic trophic pathways in a subalpine lake. Canadian Journal of Fisheries and Aquatic Sciences, 2006, 63(12): 2608-2620. DOI:10.1139/f06-148 |
| [24] |
Liboriussen L, Jeppesen E. Temporal dynamics in epipelic, pelagic and epiphytic algal production in a clear and a turbid shallow lake. Freshwater Biology, 2003, 48(3): 418-431. DOI:10.1046/j.1365-2427.2003.01018.x |
| [25] |
Glaholt Jr SP. Ecosystem response to benthic derived nutrient subsidies from omnivorous fishDissertation]. Miami: Miami University, 2003.
|
| [26] |
Guariento RD, Carneiro LS, Caliman A et al. Interactive effects of omnivorous fish and nutrient loading on net productivity regulation of phytoplankton and periphyton. Aquatic Biology, 2010, 10(3): 273-282. DOI:10.3354/ab00287 |
| [27] |
Zhang XF, Liu ZW, Jeppesen E et al. Effects of benthic-feeding common carp and filter-feeding silver carp on benthic-pelagic coupling: Implications for shallow lake management. Ecological Engineering, 2016, 88: 256-264. DOI:10.1016/j.ecoleng.2015.12.039 |
| [28] |
Zambrano L, Martínez-Meyer E, Menezes N et al. Invasive potential of common carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus) in American freshwater systems. Canadian Journal of Fisheries and Aquatic Sciences, 2006, 63(9): 1903-1910. DOI:10.1139/f06-088 |
| [29] |
Lèveque C. Out of Africa: The success story of tilapias. Environmental Biology of Fishes, 2002, 64(4): 461-464. DOI:10.1023/A:1016190529697 |
| [30] |
Pujoni DGF, Maia-Barbosa PM, Barbosa FAR et al. Effects of food web complexity on top-down control in tropical lakes. Ecological Modelling, 2016, 320: 358-365. DOI:10.1016/j.ecolmodel.2015.10.006 |
| [31] |
Yu JL, Liu ZW, He H et al. Submerged macrophytes facilitate dominance of omnivorous fish in a subtropical shallow lake: Implications for lake restoration. Hydrobiologia, 2016, 775(1): 97-107. DOI:10.1007/s10750-016-2717-7 |
| [32] |
Wootton KL. Omnivory and stability in freshwater habitats: Does theory match reality?. Freshwater Biology, 2017, 62(5): 821-832. DOI:10.1111/fwb.12908 |
| [33] |
Zhang XF, Mei XY, Gulati RD. Effects of omnivorous tilapia on water turbidity and primary production dynamics in shallow lakes: Implications for ecosystem management. Reviews in Fish Biology and Fisheries, 2017, 27(1): 245-254. DOI:10.1007/s11160-016-9458-6 |
| [34] |
Turker H, Eversole AG, Brune DE. Filtration of green algae and cyanobacteria by Nile tilapia, Oreochromis niloticus, in the Partitioned Aquaculture System. Aquaculture, 2003, 215(1/2/3/4): 93-101. DOI:10.1016/S0044-8486(02)00133-3 |
| [35] |
Yang K, Zhang XF, Liu ZW. Effect of Tilapia on phytoplankton community. Journal of Hydroecology, 2010, 3(3): 12-17. [杨凯, 张修峰, 刘正文. 罗非鱼对浮游植物群落的影响. 水生态学杂志, 2010, 3(3): 12-17. DOI:10.15928/j.1674-3075.2010.03.024] |
| [36] |
Wang YP, Zhao Y, Zeng QF et al. Changes in the photosynthetic activity of Microcystis colonies after gut passage through omnivorous fish. China Environmental Science, 2013, 33(3): 524-529. [王银平, 赵勇, 曾庆飞等. 杂食性鱼类排泄物中藻类光能活性研究. 中国环境科学, 2013, 33(3): 524-529. DOI:10.3969/j.issn.1000-6923.2013.03.021] |
| [37] |
Figueredo CC, Giani A. Ecological interactions between Nile Tilapia (Oreochromis niloticus L.) and the phytoplanktonic community of the Furnas Reservoir (Brazil). Freshwater Biology, 2005, 50(8): 1391-1403. DOI:10.1111/j.1365-2427.2005.01407.x |
| [38] |
Menezes RF, Attayde JL, Vasconcelos RF. Effects of omnivorous filter-feeding fish and nutrient enrichment on the plankton community and water transparency of a tropical reservoir. Freshwater Biology, 2010, 55(4): 767-779. DOI:10.1111/j.1365-2427.2009.02319.x |
| [39] |
Lu KH, Jin CH, Wang YC. Control of cyanobacterial blooms in eutrophication lakes by tilapia. Journal of Fisheries of China, 2005, 29(6): 811-818. [陆开宏, 金春华, 王扬才. 罗非鱼对蓝藻的摄食消化及对富营养化水体水华的控制. 水产学报, 2005, 29(6): 811-818.] |
| [40] |
Lu KH, Jin CH, Dong SL et al. Feeding and control of blue-green algal blooms by tilapia (Oreochromis niloticus). Hydrobiologia, 2006, 568(1): 111-120. DOI:10.1007/s10750-006-0023-5 |
| [41] |
van Dam AA, Beveridge MC, Azim ME et al. The potential of fish production based on periphyton. Reviews in Fish Biology and Fisheries, 2002, 12(1): 1-31. DOI:10.1023/A:1022639805031 |
| [42] |
Starling F, Lazzaro X, Cavalcanti C et al. Contribution of omnivorous tilapia to eutrophication of a shallow tropical reservoir: Evidence from a fish kill. Freshwater Biology, 2002, 47(12): 2443-2452. DOI:10.1046/j.1365-2427.2002.01013.x |
| [43] |
Vanni MJ. Nutrient cycling by animals in freshwater ecosystems. Annual Review of Ecology and Systematics, 2002, 33(1): 341-370. DOI:10.1146/annurev.ecolsys.33.010802.150519 |
| [44] |
Haertel-Borer SS, Allen DM, Dame RF. Fishes and shrimps are significant sources of dissolved inorganic nutrients in intertidal salt marsh creeks. Journal of Experimental Marine Biology and Ecology, 2004, 311(1): 79-99. DOI:10.1016/j.jembe.2004.05.002 |
| [45] |
Garcia-Berthou E. Size- and depth-dependent variation in habitat and diet of the common carp (Cyprinus carpio). Aquatic Sciences, 2001, 63(4): 466-476. DOI:10.1007/s00027-001-8045-6 |
| [46] |
Panek FM. Biology and ecology of carp. In: Cooper EL ed. Carp in North America. Bethesda: American Fisheries Society, 1987: 1-15.
|
| [47] |
He H, Hu E, Yu JL et al. Does turbidity induced by Carassius carassius limit phytoplankton growth? A mesocosm study. Environmental Science and Pollution Research, 2017, 24(5): 5012-5018. DOI:10.1007/s11356-016-8247-z |
| [48] |
Yu JL, Xia ML, Kong M et al. A small omnivorous bitterling fish (Acheilognathus macropterus) facilitate dominance of cyanobacteria, rotifers and Limnodrilus in an outdoor mesocosm experiment. Environmental Science and Pollution Research, 2020, 27(19): 23862-23870. DOI:10.1007/s11356-020-08774-5 |
| [49] |
Sutela T, Vehanen T, Huusko A et al. Seasonal shift in boreal riverine fish assemblages and associated bias in bioassessment. Hydrobiologia, 2017, 787(1): 193-203. DOI:10.1007/s10750-016-2959-4 |
| [50] |
Jeppesen E, Meerhoff M, Holmgren K et al. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia, 2010, 646(1): 73-90. DOI:10.1007/s10750-010-0171-5 |
| [51] |
González-Bergonzoni I, Meerhoff M, Davidson TA et al. Meta-analysis shows a consistent and strong latitudinal pattern in fish omnivory across ecosystems. Ecosystems, 2012, 15(3): 492-503. DOI:10.1007/s10021-012-9524-4 |
 2021, Vol. 33
2021, Vol. 33 


