水域生态系统中硫的转化与有机质成岩埋藏、营养元素循环、水体酸化、重金属生物有效性等紧密耦合,产生重要的环境效应[1-4]. 有机硫(organic sulfur,Sorg)包括由硫酸盐同化还原形成的生物有机硫(Sbio)和由有机质硫化形成的成岩有机硫(Sdiag),是湖泊沉积物中硫的主要赋存形态之一,占比可高达90 %[5-6]. 生物有机硫和成岩有机硫中的还原态硫功能团均可络合Hg+、Cu2+、Pb2+、Ag+及As3+等金属离子,对有毒有害元素的形态和生物有效性具有重要的抑制作用[7]. 成岩有机硫的形成可提高活性有机质的稳定性及其在沉积物中的埋藏保存,而且硫化过程记录了氧化还原条件和元素生物地球化学循环等重要的环境信息[8]. Sinninghe Damsté等[9]较早提出有机质成岩埋藏的自然硫化机制,即S2-优先与铁结合形成铁硫化物,当S2-含量超过铁硫化物计量配比时,便与有机质结合硫化形成有机硫,这主要发生在有机质早期成岩阶段. 湖泊沉积物中的活性有机质在好氧和厌氧条件下可降解形成温室气体二氧化碳和甲烷[10],因此,开展硫转化及其与有机质结合、调控的相关研究对认识区域碳、硫循环及潜在的气候环境效应具有重要意义.
活性铁是指能被1 mol/L盐酸提取的铁,主要包括无定形或弱晶型Fe(Ⅲ)氧化物和Fe(Ⅱ)(黄铁矿除外)以及Fe3S4(含量很少,可忽略不计)[11]. 活性铁与硫化物可发生铁的硫化作用,生成酸可挥发性硫(acid volatile sulfur, AVS)和黄铁矿硫(pyrite sulfur, CRS). AVS和CRS是沉积物中还原性无机硫(RIS)的两个主要终产物[12-14],它们的形成会抑制有机质硫化,而高含量活性铁对CRS的形成和有机质硫化则具有促进作用,使得有机质硫化受制于C-S-Fe耦合体系[15]. 黄铁矿化度(DOP)和硫化度(DOS)是评估沉积物中黄铁矿和铁硫化物的形成是受有机质还是活性铁控制的重要指标[16-17]. DOP值是表征沉积物氧化还原条件并识别黄铁矿形成的限制因素[18-19],其判断标准为DOP>0.75为缺氧环境,0.45 < DOP < 0.75为贫氧环境,DOP < 0.45为氧化环境[20]. 由于自然界中存在大量的AVS,DOP并不能完全指征铁与硫化物反应的程度,而DOS可以更好地表征铁对硫化物的限制程度.
硫同位素组成被广泛应用于水域生态系统中硫的来源及其转化循环过程研究[8, 21-23],在解析有机硫来源组成及其转化方面具有重要作用[15, 24]. 沉积物中有机硫相对于同时存在的硫化物富集34S约为10‰,但在某些条件下可达30‰以上[25-27],这主要与有机硫中生物有机硫和成岩有机硫的形成途径有关. 硫酸盐同化还原过程导致的硫同位素分馏效应仅为1‰~3‰ [28],形成的生物有机硫与其前体硫酸盐具有相似的硫同位素组成;而硫酸盐异化还原生成的硫化氢及其氧化/歧化产物中间态硫的34S亏损显著[8, 23, 29-30],成岩有机硫由硫化氢/中间态硫与有机质化合形成,其硫同位素组成继承前体硫化氢/中间态硫偏负的信号. 总有机硫硫同位素组成取决于其生物有机硫和成岩有机硫的组成比例,介于硫化物和硫酸盐之间[31]. 因此,硫同位素是研究有机硫来源组成、转化以及成岩有机硫与铁硫化物联系的重要手段.
南极菲尔德斯半岛和阿德雷岛位于全球变暖最快的南极半岛西北部,随着冰川后退、陆地出露和雪冰消融,沿海岛屿形成了众多湖泊和集水区;其中,阿德雷岛一些湖泊接受了企鹅活动带来的大量有机质和营养元素[32],成为温室气体重要的排放源. 我们前期对阿德雷岛受企鹅活动影响的Y2湖和未受影响的菲尔德斯半岛燕鸥(YO)湖沉积物中硫和重金属形态组成的分析研究[33-34]表明,有机硫是Y2湖沉积物中硫的主要赋存形态,企鹅活动带来的高含量有机质驱动了强烈的硫酸盐还原,进而限制了微量重金属的生物有效性. 而有关Y2、YO湖沉积物有机硫来源的具体组成及其与有机质、铁硫化物的联系等还不清楚. 因此,本研究在前期工作的基础上,利用总有机硫硫同位素组成、活性铁的DOP和DOS解析并揭示南极湖泊有机硫的来源组成及其与铁硫化物间的联系,对认识湖泊硫生物地球化学转化及其与有机质成岩埋藏、重金属生物有效性的关联提供了视角,并为进一步开展南极气候变化敏感区湖泊硫转化的气候环境效应研究奠定了基础.
1 材料与方法 1.1 研究区域与样品采集南极菲尔德斯半岛和阿德雷岛位于乔治王岛西南部,面积约33 km2,是乔治王岛最大的无冰区,也是中国南极科学考察站长城站所在地. 当地气候寒冷,年平均气温为-2.2℃. 菲尔德斯半岛和阿德雷岛上的大部分湖泊是由冰川活动形成的,其中阿德雷岛Y2湖位于该岛西部,为一小型月牙状淡水湖,面积约3500 m2,水深在0.5 m左右,海拔高度约12 m,南侧邻海,地势东高西低. Y2湖周边历史时期有企鹅活动,湖泊沉积物中含有大量企鹅转移而来的有机质、营养盐和重金属污染物[32];而菲尔德斯半岛的YO湖位于中国南极长城站东南方向,为一现代小型湖泊,面积约9000 m2,距离长城湾最近的海岸线约200 m;YO湖未受企鹅活动影响,营养元素含量较低.
本研究分析的Y2、YO湖沉积物为Chen等[33]研究的平行样. 样品于中国第29次南极科学考察期间(2012—2013年)分别采集于南极阿德雷岛的Y2湖和菲尔德斯半岛的YO湖(图 1). 企鹅粪土沉积剖面Y2按1 cm间隔分成60个样,原生湖泊沉积剖面YO按1 cm间隔分成30个样. 在进行化学分析之前,一部分样品-20℃冷冻保存,而另一部分样品则在去除岩石碎片和生物残体后冷冻干燥并研磨过筛(孔径为0.125 mm). 本文选择Chen等[33]硫形态研究中的16个Y2和11个YO沉积物序列进行分析.
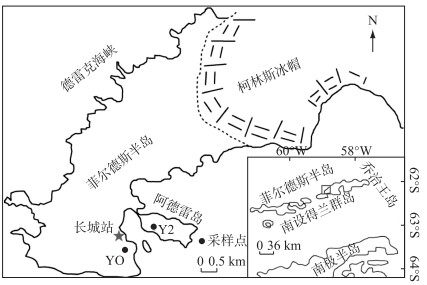
|
图 1 南极半岛乔治王岛阿德雷岛和菲尔德斯半岛的研究区和采样点(参照Chen等[34]) Fig.1 Study area and sampling sites on Fildes Peninsula and Ardley Island according to Chen et al[34] |
在氮气手套箱内称取解冻沉积物0.5 g,加入25 mL 1 mol/L HCl,在室温下振荡24 h,然后离心(6000 r/min, 15 min). 吸取25 mL上清液置于100 mL锥形瓶中,加入1 mL盐酸(1∶3)和1 mL质量分数为10 %的盐酸羟胺,煮沸至15 mL,冷却至室温后转移至100 mL容量瓶并定容,混匀后移取1~5 mL于50 mL比色管中定容,使用邻菲啰啉分光光度法测定提取液中总活性铁Fe(HCl)的含量[19],实验室内样品重复测定误差小于5 %.
1.2.2 DOP和DOS的计算黄铁矿化度(DOP)和硫化度(DOS)按下列公式[35]计算:
| ${\rm{DOP = Fe(CRS)/[Fe(CRS) + Fe(HCl)]}} $ | (1) |
| ${\rm{DOS = [Fe(AVS) + Fe(CRS)]/[Fe(CRS) + Fe(HCl)]}} $ | (2) |
式中,Fe (CRS)为黄铁矿结合态铁的含量,按FeS2的1∶2化学计量比CRS/2计算;Fe (HCl)为总活性铁含量;Fe(AVS)为假定AVS全部以FeS形式存在的硫化物结合态Fe的含量;AVS和CRS含量数据引自Chen等[33].
1.2.3 有机硫硫同位素分析前期研究中利用NaH2PO4溶液和改进的冷扩散法依次提取了沉积物中的SO42-,AVS、CRS和单质硫(elemental sulfur, ES)[33]. 提取后的AVS和CRS中加入足量AgNO3溶液转化为Ag2S,用于硫同位素分析[36]. 本研究将提取了SO42-、AVS、CRS和ES后的残余沉积物用于有机硫硫同位素样品制备. 沉积物用蒸馏水洗涤3次,然后转移至烧杯中烘干、研磨;取定量粉末样与其3倍质量的艾氏卡试剂(质量比轻质MgO∶Na2CO3=2∶1)混合均匀,置于瓷坩埚中并覆盖一层艾氏卡试剂;将坩埚置于马弗炉中800℃焙烧2 h,样品中有机硫全部转化为硫酸钡;待样品冷却后,加300 mL蒸馏水加热30 min溶解并经0.2 μm滤膜过滤;用HCl调节滤液pH < 2.0 (防止铁水解),加10 mL 10 %BaCl2,于60℃反应12 h后过滤、收集硫酸钡并干燥至恒重. 在矿床地球化学国家重点实验室采用稳定同位素比值质谱仪(MAT253-Flash EA 2000)测定硫酸钡硫同位素组成,分析精度为0.2‰. 测试过程中使用VCDT标准IAEA-SO-5(+0.5‰),IAEA-SO-6(-34.1‰)和NBS-127 (+20.3‰)作为标样,结果以δ (‰)表示,其公式为:δ34S (‰)=[(Rsample-Rstandard)/Rstandard]×1000.
1.2.4 有机硫源占比计算沉积物中Sorg由Sbio和Sdiag按一定比例组成,根据同位素质量平衡按公式(3)计算两种硫源的组成比例.
| $f\cdot{{\delta }^{34}}{{\rm{S}}_{{\rm{diag}}}} + (1 - f)\cdot{{\delta}^{{\rm{34}}}}{{\rm{S}}_{{\rm{bio}}}} = {{\delta}^{{\rm{34}}}}{{\rm{S}}_{{\rm{org}}}} $ | (3) |
式中,f代表成岩有机硫占总有机硫的比例(Sdiag/Sorg),1-f代表生物有机硫占总有机硫的比例(Sbio/Sorg);δ34Sdiag和δ34Sbio分别是成岩有机硫和生物有机硫的硫同位素组成,δ34Sorg是沉积物总有机硫硫同位素组成. Y2沉积物中生物有机硫来源于海洋源的企鹅粪,与海水硫酸盐有相似的硫同位素组成,YO硫酸盐主要来自海盐飞沫[33],其生物有机硫也具有海水硫酸盐硫同位素组成信号,即δ34Sbio≈20‰ [37]. 而有机质硫化形成的成岩有机硫硫同位素继承了硫酸盐异化还原产物硫化氢的硫同位素组成信号,即δ34Sdiag≈δ34SAVS≈δ34SCRS. 其中,沉积物δ34SAVS和δ34SCRS数据引自Shen等[36].
2 结果Y2和YO沉积物δ34Sorg、活性铁含量、DOP和DOS的垂直分布如图 2所示. Y2剖面沉积物有机硫硫同位素组成呈3段式垂直分布特征,在1~15 cm较低(1.5‰~3.7‰,平均值为2.7‰),在19~45 cm中等且波动较小(4.1‰~5.7‰,平均值为4.7‰),在48~56 cm显著富集34S(8.5‰~11.4‰,平均值为9.6‰). YO剖面沉积物δ34Sorg除28 cm层位明显偏负外,从底部到表层呈现波动变负的趋势.

|
图 2 Y2和YO沉积物中δ34Sorg、活性铁含量、DOP、DOS和Sdiag占比的剖面变化 Fig.2 Vertical changes of δ34Sorg, reactive iron, DOP, DOS and Sdiag %in Y2 and YO sediments |
Y2沉积物中总活性铁含量范围为15.34~42.41 mg/g,平均值为25.4 mg/g,其中在8~19 cm含量较高,其他层位含量平均值为21.67 mg/g且波动较小. YO沉积物活性铁含量为4.26~5.69 mg/g,平均值为4.86 mg/g,远小于Y2. Y2沉积物DOP (0.45±0.21)远低于DOS (5.10±4.13),其DOS在8 cm层位达到较高值(11.82),在底部48~56 cm沉积物的DOP和DOS均变大且在48 cm处达到最高值(12.98). YO沉积物的DOP (0.49±0.16)和DOS (0.83±0.34)相差不大,随深度呈波动增大趋势. Y2剖面1~8 cm沉积物总有机硫中Sdiag占比均大于50 %,平均值为58.9 %;12~45 cm沉积物Sdiag的占比变化不大(42.7 %~56.2 %),平均值为49.9 %,底部48~56 cm沉积物Sdiag占比均小于50 %,均值为41.0 %. YO剖面除28 cm沉积物Sdiag占比高达95.4 %外,其他层位Sdiag占比范围为39.4 %~59.2 %,平均值为46.2 %.
3 讨论 3.1 有机硫来源组成有机硫根据其形成途径可分为生物有机硫和成岩有机硫[38-39]. 生物有机硫形成于硫酸盐同化还原,即生物摄取硫酸盐用于合成氨基酸和其他含硫细胞组分[36]. 企鹅以粪便的形式将大量生物有机硫输送到Y2湖,形成鸟粪土沉积物[32]. 鸟粪生物有机硫来源于海洋SO42-的同化还原,该过程硫同位素分馏可忽略不计[28],故鸟粪生物有机硫与海洋SO42-有相似的硫同位素组成(δ34S≈20‰),企鹅粪便的输入会导致有机硫显著富集34S. 成岩有机硫形成有机质硫化,即由硫酸盐还原产生的各种还原态硫与有机质结合生成大分子有机硫化合物,还原态硫与有机质结合过程硫同位素分馏可忽略,所以成岩有机硫与H2S有相似的δ34S值[31].
Y2剖面1~15 cm沉积物δ34Sorg值较低,指示贫34S的成岩有机硫占比较大. δ34Sorg值在8 cm处达到最小值,表明8 cm层位沉积物具有最高的有机质硫化程度. Y2剖面19~45 cm沉积物δ34Sorg变化不大,表明该层位成岩有机硫和生物有机硫组成比例稳定;Y2沉积物RIS/SO42-比值指示剖面底部48 cm硫酸盐还原很强[34],大量的还原态硫易与有机质结合形成硫同位素偏负的成岩有机硫组分,但由于该层位时段湖泊周边企鹅种群数量增加[32],大量企鹅粪的输入使沉积物中富34S的生物有机硫也快速增加;本研究Y2剖面48~56 cm沉积物显著富集的δ34Sorg指示生物有机硫比例很高,指示鸟粪带来的生物有机硫超过了由硫酸盐还原产物硫化氢/中间态硫与有机质形成的成岩有机硫的增加. 此外,研究表明沉积扰动会加速生物有机硫大分子有机物快速分解,形成更多的小分子生物有机硫[40],本研究中企鹅粪便输入沉积的物理扰动过程可能会产生类似的作用,分解释放的大量小分子Sbio可快速与有机质有效再结合,导致沉积物生物有机硫增加.
YO剖面0~8 cm沉积物δ34Sorg值升高,与先前Chen等[33]硫形态指示的明显有机硫矿化相一致,研究表明表层沉积物的微量溶解氧能促进微生物分泌一些物质并有利于有机硫的矿化[41];8~26 cm和30 cm层位沉积物δ34Sorg值变化不大,指示这些层位生物有机硫和成岩有机硫的组成比例稳定. YO剖面28 cm处沉积物δ34Sorg值显著降低,表明成岩有机硫组分显著增加;前期沉积物硫形态研究指出该层位的硫酸盐还原最强[33],产生的硫化氢易与有机质结合形成大量的成岩有机硫.
3.2 生物有机硫与成岩有机硫的占比有机质硫化成岩需要有足量的活性有机质,有机质不仅参与硫酸盐异化还原产生还原态硫,还直接与还原态硫结合形成有机硫[42-43]. Y2和YO沉积物有机硫中成岩有机硫所占的比例如图 2所示. Y2沉积物0~8 cm成岩有机硫所占的比例大于50 %,很可能是由于高活性有机质促进了成岩有机质硫化. 48~56 cm沉积物较低的成岩有机硫占比指示了大量的企鹅粪输入和沉积扰动导致生物有机硫占比的增加. YO沉积物0~26 cm和30 cm成岩有机硫的比例低于50 %,前期研究表明这些层位硫酸盐还原较弱[33],较低的硫化氢供给导致有机质硫化程度较低. YO沉积物28 cm成岩有机硫的比例高达95.4 %,这是因为28 cm处硫酸盐还原强度大[33],充足的硫化氢与沉积物中的有机物结合产生大量的成岩有机硫,其占比大幅增加.
3.3 有机质硫化与铁硫化的联系Y2剖面沉积物低DOP值和高DOS值(图 2)指示Y2容易发生铁的硫化过程;而YO剖面沉积物DOP>0.45,指示在还原环境中,大部分活性三价铁氧化物被还原为活性二价铁并有效转化为稳定的CRS. 目前,一些研究表明铁硫化物与成岩有机硫能同时形成[44-46]. 铁氧化物快速输入能提高中间态硫的产生,而这有可能同时促进有机质硫化和铁硫化物的形成[46-48]. 本研究中Y2剖面沉积物8~15 cm的低δ34Sorg值和高DOS指示该层位有机质硫化和铁的硫化同时发生,表明该层位高含量的活性铁有利于铁硫化物的形成且并促进了有机质硫化过程;Y2剖面48~56 cm沉积物高DOS值是企鹅活动带来的高有机质和强硫酸盐还原促进了铁的硫化作用,铁硫化物(包括黄铁矿)的大量形成对有机质硫化具有竞争性抑制作用[25, 42],从而降低了成岩有机硫的比例. YO剖面沉积物DOP和DOS随深度呈波动增加的趋势,即深部层位沉积物还原性无机硫更多以CRS为主;同时,28 cm处强硫酸盐还原提供了充足的硫化氢/中间态硫与有机质结合为成岩有机硫并降低δ34Sorg,成岩有机硫占比高达95.4 %,表明该层位也存在有机质硫化与CRS的形成同时发生.
硫在沉积物有机质的成岩和埋藏中发挥着重要作用[49],不同形态硫的转化会影响环境变化乃至生态安全[50-51]. 湖泊硫的转化循环涉及一系列氧化还原反应,并影响内源磷和铁的迁移释放、有机质的成岩埋藏、重金属的毒性和生物可利用性,产生重要的生态环境效应[17, 34, 52]. 环南极海岛、无冰区分布有众多接收海鸟、海兽传输的高有机质、营养盐和重金属污染的湖泊和集水区[53],这类气候敏感区水生系统水文和氧化还原条件季节性变化显著,营养盐生物地球化学转化活跃. 对南极洲沿海地区海兽排泄物的研究中发现,海兽排泄物中的有机质具有很高的生物有效性,在冻融过程中,有机质会因微生物活性升高发生矿化过程,使二氧化碳或甲烷的排放显著增加[54]. 有机质硫化过程能够提高有机质的稳定性,有效抑制有机质的矿化分解,对碳转化循环产生实质影响. 本研究结果表明,有机硫组分的来源转化与有机质、活性铁紧密耦合,对区域湖泊有机质成岩埋藏、铁硫化物形成及其对金属生物有效性限制有直接指示作用,对进一步开展营养元素生物地球化学转化的气候环境效应评估具有重要的意义.
4 结论1) Y2剖面底部生物有机硫占比的提高源于大量企鹅粪的输入,使沉积物中生物有机硫的增加超过了成岩有机硫;而YO剖面28 cm处的成岩有机硫占比的绝对优势源于强硫酸盐还原提供了充足的硫化氢/中间态硫作为有机质硫化的反应前体.
2) Y2和YO剖面部分层位沉积物有机质硫化与铁的硫化同时发生,主要与高活性铁含量和强硫酸盐还原过程相关.
致谢: 本文研究样品来源于中国极地沉积物库(合肥).
| [1] |
Yin HB, Fan CX, Ding SM et al. Distribution characteristic and correlation relationship of reactive sulfur and heavy metals in sediments of Meiliang bay and Wuli Lake of Taihu Lake. Environmental Science, 2008, 29(7): 1791-1796. [尹洪斌, 范成新, 丁士明等. 太湖梅梁湾与五里湖沉积物活性硫和重金属分布特征及相关性研究. 环境科学, 2008, 29(7): 1791-1796. DOI:10.13227/j.hjkx.2008.07.010] |
| [2] |
Liu J, Jiang T, Huang R et al. A simulation study of inorganic sulfur cycling in the water level fluctuation zone of the Three Gorges Reservoir, China and the implications for mercury methylation. Chemosphere, 2017, 166: 31-40. DOI:10.1016/j.chemosphere.2016.09.079 |
| [3] |
Zhao YP, Zhang ZQ, Wang GX et al. High sulfide production induced by algae decomposition and its potential stimulation to phosphorus mobility in sediment. Science of the Total Environment, 2019, 650: 163-172. DOI:10.1016/j.scitotenv.2018.09.010 |
| [4] |
Shen QS, Fan CX. Identification of black suspended particles in the algae-induced black bloom water column. J Lake Sci, 2015, 27(4): 591-598. [申秋实, 范成新. 藻源性湖泛水体显黑颗粒的元素形态分析与鉴定. 湖泊科学, 2015, 27(4): 591-598. DOI:10.18307/2015.0405] |
| [5] |
Morgan B, Burton ED, Rate AW. Iron monosulfide enrichment and the presence of organosulfur in eutrophic estuarine sediments. Chemical Geology, 2012, 296/297: 119-130. DOI:10.1016/j.chemgeo.2011.12.005 |
| [6] |
Wang JF, Chen JG, Dai ZH et al. Sulfur speciation in the surface sediments of lakes from different regions, China: Characterization by S K-edge XANES spectroscopy. Journal of Chemistry, 2016, 2016: 1-9. DOI:10.1155/2016/3672348 |
| [7] |
Hoffmann M, Mikutta C, Kretzschmar R. Bisulfide reaction with natural organic matter enhances arsenite sorption: Insights from X-ray absorption spectroscopy. Environmental Science & Technology, 2012, 46(21): 11788-11797. DOI:10.1021/es302590x |
| [8] |
Amrani A. Organosulfur compounds: Molecular and isotopic evolution from biota to oil and gas. Annual Review of Earth and Planetary Sciences, 2014, 42(1): 733-768. DOI:10.1146/annurev-earth-050212-124126 |
| [9] |
Sinninghe Damsté JS, Rijpstra WIC, Kock-van Dalen AC et al. Quenching of labile functionalised lipids by inorganic sulphur species: Evidence for the formation of sedimentary organic sulphur compounds at the early stages of diagenesis. Geochimica et Cosmochimica Acta, 1989, 53(6): 1343-1355. DOI:10.1016/0016-7037(89)90067-7 |
| [10] |
Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiology and Molecular Biology Reviews, 1997, 61(2): 262-280. |
| [11] |
Mortimer RJG, Davey JT, Krom MD et al. The effect of macrofauna on porewater profiles and nutrient fluxes in the intertidal zone of the Humber estuary. Estuarine, Coastal and Shelf Science, 1999, 48(6): 683-699. DOI:10.1006/ecss.1999.0479 |
| [12] |
Kraal P, Burton ED, Bush RT. Iron monosulfide accumulation and pyrite formation in eutrophic estuarine sediments. Geochimica et Cosmochimica Acta, 2013, 122: 75-88. DOI:10.1016/j.gca.2013.08.013 |
| [13] |
Sheng YQ, Sun QY, Shi WJ et al. Geochemistry of reduced inorganic sulfur, reactive iron, and organic carbon in fluvial and marine surface sediment in the Laizhou Bay region, China. Environmental Earth Sciences, 2015, 74(2): 1151-1160. DOI:10.1007/s12665-015-4101-8 |
| [14] |
Sun QY, Sheng YQ, Yang J et al. Dynamic characteristics of sulfur, iron and phosphorus in coastal polluted sediments, North China. Environmental Pollution, 2016, 219: 588-595. DOI:10.1016/j.envpol.2016.06.019 |
| [15] |
Werne J, Hollander D, Lyons T et al. Organic sulfur biogeochemistry: recent advances and future research directions. In: Amend JP, Edwards KJ, Lyons TW eds. Sulfur biogeochemistry-past and present: Boulder, Colorado, Geological Society of America Special 2004, 379: 117-134. DOI: 10.1130/0-8137-2379-5.135.
|
| [16] |
Burton ED, Bush RT, Johnston SG et al. Sulfur biogeochemical cycling and novel Fe-S mineralization pathways in a tidally re-flooded wetland. Geochimica et Cosmochimica Acta, 2011, 75(12): 3434-3451. DOI:10.1016/j.gca.2011.03.020 |
| [17] |
Jiang M, Sheng YQ, Liu QQ et al. Conversion mechanisms between organic sulfur and inorganic sulfur in surface sediments in coastal rivers. Science of the Total Environment, 2021, 752: 141829. DOI:10.1016/j.scitotenv.2020.141829 |
| [18] |
Morse JW, Rickard D. Chemical dynamics of sedimentary acid volatile sulfide. Environmental Science & Technology, 2004, 38(7): 131A-136A. DOI:10.1021/es040447y |
| [19] |
Jiang M, Zhao GQ, Li ZR et al. Distribution characteristics and relationship between reduced inorganic sulfur and reactive iron in core sediments outside the mouth of the Jiahe River in Yantai. Marine Sciences, 2018, 42(8): 90-97. [姜明, 赵国强, 李兆冉等. 烟台夹河口外柱状沉积物还原性无机硫、活性铁的变化特征及其相互关系. 海洋科学, 2018, 42(8): 90-97. DOI:10.11759/hykx20180313001] |
| [20] |
Canfield DE, Thamdrup B. Fate of elemental sulfur in an intertidal sediment. FEMS Microbiology Ecology, 1996, 19(2): 95-103. DOI:10.1016/0168-6496(95)00083-6 |
| [21] |
Zhu MX, Shi XN, Yang GP et al. Formation and burial of pyrite and organic sulfur in mud sediments of the East China Sea inner shelf: Constraints from solid-phase sulfur speciation and stable sulfur isotope. Continental Shelf Research, 2013, 54: 24-36. DOI:10.1016/j.csr.2013.01.002 |
| [22] |
Kang PG, Mitchell MJ, Mayer B et al. Isotopic evidence for determining the sources of dissolved organic sulfur in a forested catchment. Environmental Science & Technology, 2014, 48(19): 11259-11267. DOI:10.1021/es502563n |
| [23] |
Fike DA, Bradley AS, Rose CV. Rethinking the ancient sulfur cycle. Annual Review of Earth and Planetary Sciences, 2015, 43(1): 593-622. DOI:10.1146/annurev-earth-060313-054802 |
| [24] |
Brüchert V. Early diagenesis of sulfur in estuarine sediments: The role of sedimentary humic and fulvic acids. Geochimica et Cosmochimica Acta, 1998, 62(9): 1567-1586. DOI:10.1016/S0016-7037(98)00089-1 |
| [25] |
Anderson TF, Pratt LM. Isotopic evidence for the origin of organic sulfur and elemental sulfur in marine sediments. ACS Symposium Series. Washington, DC: American Chemical Society, 1995: 378-396. DOI:10.1021/bk-1995-0612.ch021
|
| [26] |
Bottrell SH, Hatfield D, Bartlett R et al. Concentrations, sulfur isotopic compositions and origin of organosulfur compounds in pore waters of a highly polluted raised peatland. Organic Geochemistry, 2010, 41(1): 55-62. DOI:10.1016/j.orggeochem.2009.07.005 |
| [27] |
Shawar L, Halevy I, Said-Ahmad W et al. Dynamics of pyrite formation and organic matter sulfurization in organic-rich carbonate sediments. Geochimica et Cosmochimica Acta, 2018, 241: 219-239. DOI:10.1016/j.gca.2018.08.048 |
| [28] |
Sela-Adler M, Said-Ahmad W, Sivan O et al. Isotopic evidence for the origin of dimethylsulfide and dimethylsulfoniopropionate-like compounds in a warm, monomictic freshwater lake. Environmental Chemistry, 2016, 13(2): 340. DOI:10.1071/en15042 |
| [29] |
Habicht KS, Canfield DE. Sulfur isotope fractionation during bacterial sulfate reduction in organic-rich sediments. Geochimica et Cosmochimica Acta, 1997, 61(24): 5351-5361. DOI:10.1016/S0016-7037(97)00311-6 |
| [30] |
Brüchert V, Knoblauch C, Jørgensen BB. Controls on stable sulfur isotope fractionation during bacterial sulfate reduction in Arctic sediments. Geochimica et Cosmochimica Acta, 2001, 65(5): 763-776. DOI:10.1016/S0016-7037(00)00557-3 |
| [31] |
Aizenshtat Z, Amrani A. Significance of δ34S and evaluation of its imprint on sedimentary organic matter: I. The role of reduced sulfur species in the diagenetic stage: A conceptual review. In: Hill RJ, Leventhal J et al eds. The geochemical society special publications. Amsterdam: Elsevier, 2004: 15-33. DOI: 10.1016/s1873-9881(04)80004-x.
|
| [32] |
Sun LG, Xie ZQ, Zhao JL. A 3, 000-year record of penguin populations. Nature, 2000, 407(6806): 858. DOI:10.1038/35038163 |
| [33] |
Chen YQ, Shen LL, Huang T et al. Transformation of sulfur species in lake sediments at Ardley Island and Fildes Peninsula, King George Island, Antarctic Peninsula. Science of the Total Environment, 2020, 703: 135591. DOI:10.1016/j.scitotenv.2019.135591 |
| [34] |
Chen YQ, Ge JW, Huang T et al. Restriction of sulfate reduction on the bioavailability and toxicity of trace metals in Antarctic lake sediments. Marine Pollution Bulletin, 2020, 151: 110807. DOI:10.1016/j.marpolbul.2019.110807 |
| [35] |
Berner RA, Scott MR, Thomlinson C. Carbonate alkalinity in the pore waters of anoxic marine sediments1. Limnology and Oceanography, 1970, 15(4): 544-549. DOI:10.4319/lo.1970.15.4.0544 |
| [36] |
Shen L, Huang T, Chen Y et al. Diverse transformations of sulfur in seabird-affected sediments revealed by microbial and stable isotope analysis. Manuscript submitted, 2021.
|
| [37] |
Kaplan IR, Rittenberg SC. Microbiological fractionation of sulphur isotopes. Journal of General Microbiology, 1964, 34: 195-212. DOI:10.1099/00221287-34-2-195 |
| [38] |
Huang XL, Zhu MX, Chen LJ et al. Sources and formation mechanisms of organic sulfur in Jiaozhou Bay sediments. Acta Oceanologica Sinica, 2014, 36(6): 50-57. [黄香利, 朱茂旭, 陈良进等. 胶州湾沉积物中有机硫来源及成因机制的研究. 海洋学报: 中文版, 2014, 36(6): 50-57.] |
| [39] |
Chen LJ, Zhu MX, Huang XL et al. Speciation of organic sulfur in inner shelf sediments of the East China Sea: Evidence from K-edge XANES spectroscopy. Geochimica, 2015, 44(1): 61-70. [陈良进, 朱茂旭, 黄香利等. 东海内陆架沉积物中有机硫形态的K边XANES谱分析. 地球化学, 2015, 44(1): 61-70. DOI:10.19700/j.0379-1726.2015.01.005] |
| [40] |
Werne JP, Lyons TW, Hollander DJ et al. Investigating pathways of diagenetic organic matter sulfurization using compound-specific sulfur isotope analysis. Geochimica et Cosmochimica Acta, 2008, 72(14): 3489-3502. DOI:10.1016/j.gca.2008.04.033 |
| [41] |
Li ST, Lin B, Zhou W. Soil organic sulfur mineralization in the presence of growing plants under aerobic or waterlogged conditions. Soil Biology and Biochemistry, 2001, 33(6): 721-727. DOI:10.1016/S0038-0717(00)00205-4 |
| [42] |
Hartgers WA, Lòpez JF, Sinninghe Damsté JS et al. Sulfur-binding in recent environments: II. Speciation of sulfur and iron and implications for the occurrence of organo-sulfur compounds. Geochimica et Cosmochimica Acta, 1997, 61(22): 4769-4788. DOI:10.1016/S0016-7037(97)00279-2 |
| [43] |
Cao A. The characteristic of reduced inorganic sulfur species in the Yangtze River Estuary and its environmental significance[Dissertation]. Shanghai: Fudan University, 2010. [曹爱丽. 长江口滨海沉积物中无机硫的形态特征及其环境意义[学位论文]. 上海: 复旦大学, 2010. ]
|
| [44] |
Brüchert V, Pratt LM. Contemporaneous early diagenetic formation of organic and inorganic sulfur in estuarine sediments from St. Andrew Bay, Florida, USA. Geochimica et Cosmochimica Acta, 1996, 60(13): 2325-2332. DOI:10.1016/0016-7037(96)00087-7 |
| [45] |
Urban NR, Ernst K, Bernasconi S. Addition of sulfur to organic matter during early diagenesis of lake sediments. Geochimica et Cosmochimica Acta, 1999, 63(6): 837-853. DOI:10.1016/S0016-7037(98)00306-8 |
| [46] |
Filley TR, Freeman KH, Wilkin RT et al. Biogeochemical controls on reaction of sedimentary organic matter and aqueous sulfides in Holocene sediments of Mud Lake, Florida. Geochimica et Cosmochimica Acta, 2002, 66(6): 937-954. DOI:10.1016/S0016-7037(01)00829-8 |
| [47] |
Wu SJ, Wang X, Ji QY et al. Iron-sulfur distribution and its environmental significance in three typical areas of western Lake Taihu. J Lake Sci, 2019, 31(4): 950-960. [吴松峻, 汪旋, 季秋忆等. 太湖西岸典型区域沉积物的硫铁分布特征及环境意义. 湖泊科学, 2019, 31(4): 950-960. DOI:10.18307/2019.0408] |
| [48] |
Li YC, Yu S, Strong J et al. Are the biogeochemical cycles of carbon, nitrogen, sulfur, and phosphorus driven by the "FeIII-FeII redox wheel" in dynamic redox environments?. Journal of Soils and Sediments, 2012, 12(5): 683-693. DOI:10.1007/s11368-012-0507-z |
| [49] |
Qin SS, Zhu MX, Yang GP et al. Atypical diagenesis of sulfur and iron in sediments of the river-dominated Bohai Sea (China). Journal of Marine Systems, 2019, 189: 116-126. DOI:10.1016/j.jmarsys.2018.10.004 |
| [50] |
Oueslati W, Helali MA, Zaaboub N et al. Sulfide influence on metal behavior in a polluted southern Mediterranean lagoon: Implications for management. Environmental Science and Pollution Research, 2018, 25(3): 2248-2264. DOI:10.1007/s11356-017-0529-6 |
| [51] |
Jørgensen BB, Findlay AJ, Pellerin A. The biogeochemical sulfur cycle of marine sediments. Frontiers in Microbiology, 2019, 10: 849. DOI:10.3389/fmicb.2019.00849 |
| [52] |
Wu SJ, Zhao YP, Chen YY et al. Sulfur cycling in freshwater sediments: A cryptic driving force of iron deposition and phosphorus mobilization. Science of the Total Environment, 2019, 657: 1294-1303. DOI:10.1016/j.scitotenv.2018.12.161 |
| [53] |
Sun LG, Emslie SD, Huang T et al. Vertebrate records in polar sediments: Biological responses to past climate change and human activities. Earth-Science Reviews, 2013, 126: 147-155. DOI:10.1016/j.earscirev.2013.08.004 |
| [54] |
Zhu RB, Liu YS, Ma ED et al. Greenhouse gas emissions from penguin guanos and ornithogenic soils in coastal Antarctica: Effects of freezing-thawing cycles. Atmospheric Environment, 2009, 43(14): 2336-2347. DOI:10.1016/j.atmosenv.2009.01.027 |
 2022, Vol. 34
2022, Vol. 34 

