(2: 中国科学院大学,北京 100049)
(3: 南京工业大学,环境科学与工程学院,南京 211816)
(4: 重庆三峡学院,环境与化学工程学院,重庆 404020)
(2: University of Chinese Academy of Sciences, Beijing 100049, P.R.China)
(3: School of Environmental Science and Engineering, Nanjing Tech University, Nanjing 211816, P.R.China)
(4: School of Environmental and Chemical Engineering, Chongqing Three Gorges University, Chongqing 404020, P.R.China)
铁(氢)氧化物在驱动水生生态系统中溶解性有机质(DOM)和无机磷(DIP)的生物地球化学循环过程中具有重要作用[1]. DOM主要由碳、氢、氧组成,包含氮、磷和硫等元素[2],是含有多糖、蛋白质、脂肪族、芳香族和酚类等物质的混合物,富含苯环、羧基、酚羟基等官能团,分子量在几百至100000 Da之间[2-3]. 在内陆水体中,溶解性有机碳(DOC)浓度一般在0.3~100 mg/L范围内[4],DOM中蕴含的DOC占水体中总碳的50 % 左右[5],是内陆水生态系统中碳库的重要组成部分[6]. 有机碳与铁(氢)氧化物两者的生物地球化学循环过程紧密相连. 在沉积物中,研究发现21.5 % 的有机碳直接与铁(氢)氧化物相结合[1]. 铁(氢)氧化物能通过吸附、共沉淀等过程固定有机碳,分馏DOM组分,改变水环境中有机质的种类和组成,进而对水生生态系统的能量流动、营养元素的物质循环、有机质的矿化等产生重要影响[5]. 磷是生物不可或缺的营养元素,同时也是影响湖库等内陆水体富营养化的关键因子之一[7]. 湖库中上覆水体的DIP浓度一般在0.005~0.05 mg/L之间,但沉积物间隙水中的DIP浓度可高达2 mg/L以上[8]. 湖泊沉积物富集了大量外源输入的磷,在外源磷负荷有效控制后,内源磷的释放仍可在长期维持湖泊的富营养化状态[9-10]. 铁结合态磷(Fe-P)是沉积物中磷的重要组成部分,因其在易迁移转化态磷中占比较高,且易受氧化还原环境影响而发生迁移转化,被认为是导致湖泊内源磷释放的关键因素[11-13]. 淡水水体中的铁浓度一般不超过1 mg/L [14],而在厌氧环境的沉积物间隙水中可达6 mg/L以上[15]. 还原环境下沉积物中与铁耦合的有机质和磷等伴随着铁的溶解,释放大量的二价铁Fe(Ⅱ)、DIP和DOM进入间隙水中,进而扩散到上覆水,并在氧化环境下伴随着铁的沉淀重新进入沉积物中.
在铁(氢)氧化物对DOM和DIP的固定过程中,DOM和DIP因竞争等作用产生相互影响[16-18]. 例如DIP通过占据铁(氢)氧化物表面的吸附位点、改变铁离子的水解形态等途径,影响铁(氢)氧化物对DOM的吸附分馏过程[19-22]. 另外,DOM也会通过与铁离子络合、占据吸附点位、促进铁(氢)氧化物溶解等途径,改变铁(氢)氧化物的存在形态及其对DIP的吸附固定过程. 已有研究表明,铁(氢)氧化物、DOM和DIP三者之间的相互作用会对碳的埋藏和分馏、沉积物磷的释放、铁的赋存形式等产生重要影响[23-27]. 吴文丽等综述了有机质-金属离子/氧化物-磷复合物在水体环境中的赋存状态、分离方法、稳定性及生物有效性[28],但其侧重点在于水体中溶解性的三元复合物及磷的生物有效性,没有探析金属离子/氧化物对DOM的固定分馏机制及DOM与磷之间的相互影响. Kleber等系统全面地综述了矿物-有机质之间的相互作用,但没有涉及DOM与DIP之间的相互影响[29],而该作用在水生态环境中十分重要. 目前关于三者相互作用的研究还较零散,缺乏系统全面的梳理.
基于以上背景,本文试图从铁(氢)氧化物对DOM的固定和分馏、铁(氢)氧化物对DIP的固定、DIP和DOM在铁(氢)氧化物上的相互作用以及影响因素等几个方面,综述DOM-铁(氢)氧化物-DIP(DOM-Fe-DIP)三者之间的相互作用过程,探讨其对湖泊等内陆水体生态系统的影响,并对今后的研究方向做出展望.
1 铁(氢)氧化物对溶解性有机质的固定和分馏铁(氢)氧化物对DOM的固定分馏主要通过吸附和共沉淀两种作用方式实现[23, 30]. 吸附过程形成的复合物中,DOM固定在铁(氢)氧化物表面; 而基于共沉淀过程,铁和有机质通过共沉淀螯合,有机分子会镶嵌在水铁矿晶体的孔隙内[31-32],或者通过铁离子或铁(氢)氧化物而一层层“粘”在一起(图 1)[33].
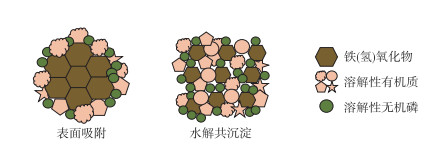
|
图 1 基于吸附和共沉淀形成的溶解性有机质-铁(氢)氧化物-溶解性无机磷复合物的示意图 Fig.1 Schematic diagram of DOM-iron (hydr)oxides-DIP complex formed based on adsorption and coprecipitation |
铁(氢)氧化物,尤其是弱结晶性和高比表面积的铁氧化物(如水铁矿(FeOOH)),是环境中普遍存在的DOM吸附剂,其最大吸附容量通常超过其他主要吸附剂(如铝硅酸盐黏土等)[24, 34]. 吸附是一个物理化学过程,可以使得有机质固定在矿物表面,防止或者延缓微生物对有机质的降解[23, 35]. 铁(氢)氧化物表面的羟基可通过配体交换吸附带有负电荷的有机质[34]. 此外,铁离子还可以通过形成金属桥键连接带负电的阴离子(如砷酸根、磷酸根等)和DOM[22, 36-37]. 铁(氢)氧化物对DOM的固定能力与其自身性质(如比表面积、孔隙结构等)、DOM的组成及其与铁(氢)氧化物的结合方式、水化学环境如pH和离子强度等有关. 铁(氢)氧化物会随时间发生老化从而降低了其吸附能力,如弱结晶的水铁矿在老化过程中转变为纤铁矿和针铁矿,金属矿物的稳定性逐渐增加,而其对DOM的吸附能力逐渐降低[38]. 高离子强度可以通过掩盖有机质分子的负电荷,降低有机质分子之间和内部的静电排斥力,使有机质分子更紧密的聚集在吸附剂上,从而增强矿物对有机质的吸附[39]. 同样,较低的pH也有类似的作用,通过质子化掩盖DOM上的负电荷,降低其溶解度,增强其对矿物表面的亲和力[22, 26]. 同时,较低的pH还会改变矿物表面形态,使其表面带正电荷,从而诱导带负电荷的有机分子吸附并促进表面络合物的形成[40]. 一般而言,随着pH值的增加,矿物对DOM的吸附能力下降[41-42]. 除了静电吸附和配体交换外,有研究发现当矿物表面处于中性时,通过熵驱动的疏水排斥现象以及范德华力和氢键的形成,非极性有机分子可以在矿物表面积累[20, 31].
DOM与铁的共沉淀过程一般在pH和氧化还原环境变化频繁的环境中容易发生[30, 43-44],如浅水湖泊的沉积物-水界面、湿地、暂时性淹水的水稻土等. 共沉淀是沉积物中铁和有机碳结合的重要方式,厌氧条件下沉积物或间隙水中的Fe(Ⅱ)释放进入上覆水,在氧化环境条件下,重新氧化为三价铁(Fe(Ⅲ))并发生水解生成铁(氢)氧化物,通过吸附包裹等作用发生铁与DOM的混凝共沉淀过程[32-34]. 不同于吸附后的复合物,通过共沉淀形成的复合物可以固定更多的有机碳. 研究表明铁(氢)氧化物对有机碳的吸附过程产生的复合物的碳铁摩尔比最大值为1,而通过共沉淀产生的复合物碳铁摩尔比可达6~10[1, 45]. Lalonde等提取了湖泊、海洋、海口等生境沉积物中的铁和有机碳,发现沉积物中的有机碳和铁的摩尔比平均值为4.0±2.8,远远超过了铁(氢)氧化物对有机碳的最大吸附能力,而与有机碳和铁通过共沉淀螯合形成的复合物的碳铁比一致[1].
由于DOM具有复杂的物质组成和结构,铁(氢)氧化物在对其吸附/共沉淀固定过程中往往表现出选择性吸附特征,使得DOM在矿物-水界面组分发生变化,即DOM的分馏[24, 42, 46-48]. Sowers等研究发现铁(氢)氧化物优先吸附芳香族和含羧基组分,并造成不含羧基的脂肪族化合物在溶液中的富集[24]; Coward等研究发现芳香族化合物优先被铁(氢)氧化物吸附,其后是木质素类和脂肪族化合物[46]; 在氧化环境条件下,铁离子氧化生成铁(氢)氧化物,并与DOM通过共沉淀等过程,去除沉积物-水界面处大量的DOC,且维管植物衍生的芳香化合物和热解化合物优先被固定沉淀,而大多数富含羧基的脂肪酸仍保留在水体中[43]. 无论吸附还是共沉淀过程,大分子量高芳香性组分优先被铁固定[42, 47],且陆源类腐殖酸组分比微生物源类腐殖酸组分与铁的亲和力更强[47]. 值得注意的是,尽管芳香族和多酚比脂肪族更容易被铁(氢)氧化物通过吸附和共沉淀固定分馏,但这种分馏特征在吸附过程中更加明显[49],这也意味着吸附和共沉淀过程铁与DOM的不同结合方式. 金属矿物对芳香组分的优先吸附与π-π作用有关,π-π作用为芳香组分提供了大量吸附点位,与DOM中其他组分相比,芳香组分不易受矿物表面吸附点位的限制[42],而含羧基组分的优先吸附与矿物表面的羟基与含羧基化合物的配位交换有关[24, 50]. Sollins等提出了一种“洋葱概念模型”(layer-by-layer onion),即优先被固定的DOM组分如含羧基的肽化合物或者芳香族化合物等通过配体交换等强特异性相互作用形成“洋葱”的初始内层,并提供了一个表面,脂肪族等其他化合物可以进一步附着在其上形成后续层,从而形成像洋葱一样的层状结构[33, 49]. 此外,与铁(氢)氧化物对DOM的选择性固定分馏特征类似,Fe-DOM复合物在还原释放过程中,也表现出不同DOM组分的非同步释放. 如Han等研究发现,对于吸附和共沉淀形成的Fe-DOM复合物,在Fe还原过程中,脂肪族化合物优先释放到溶液中[49]. 通过吸附或共沉淀过程引起的DOM直接或间接的分馏改变了水环境中的DOM组成及其生物可利用性,进而影响了碳的循环过程.
2 铁(氢)氧化物对溶解性无机磷的固定铁(氢)氧化物通过吸附和共沉淀过程与DIP结合(图 1),有效地促进了磷的埋藏[51]. 当环境中的pH值小于铁(氢)氧化物的零电荷点时,铁(氢)氧化物可以通过静电吸附固定DIP. 同时,DIP可以通过单齿单核、双齿单核和双齿双核的表面络合作用吸附在铁氧化物表面[52-53]. 铁盐在溶解于水中时,Fe(Ⅲ)/Fe(Ⅱ)可以直接与DIP发生共沉淀,Fe(Ⅲ)/Fe(Ⅱ)水解为铁(氢)氧化物进一步吸附DIP,从而达到水相中DIP的去除和埋藏[51, 54].
铁结合态磷(Fe-P)是沉积物中易迁移转化磷的重要组成部分[55-58]. 众多研究表明,沉积物总磷中Fe-P的占比一般在10 %~50 % 之间[11, 55, 59-62],如巢湖沉积物中Fe-P丰度为28 %~45 % [60],太湖沉积物中Fe-P丰度为10 %~40 % [63],中国华北潘家口、大黑汀、于桥梯级水库沉积物中Fe-P的丰度为10 %~30 % [55]. 然而Fe-P在氧化还原条件、pH和铁(氢)氧化物结构发生改变时容易释放. 由于铁(氢)氧化物在缺氧环境下会还原溶解,当沉积物或间隙水处于缺氧环境时,铁(氢)氧化物固定的磷会释放扩散进入上覆水,成为生物可利用性磷[11, 64]. 对于富营养化湖泊而言,间隙水中的铁和磷往往同步变化或释放进入上覆水,即铁磷耦合[15, 65]. 除氧化还原条件外,pH值也是影响铁(氢)氧化物固定DIP的关键因素. 一般而言,在适当的pH值范围内(4~10),随着pH值的升高,铁(氢)氧化物对DIP的吸附能力逐渐降低[66-67]. 这是因为pH可以改变铁(氢)氧化物的表面形态,如FeOH2+、FeOH和FeO-[51],以及磷酸根的赋存形态,如H2PO4-和HPO42-等. 铁(氢)氧化物的零电荷点(pHpzc)通常在5.4~8.6之间[68],只有当溶液中pH值小于铁(氢)氧化物的pHpzc时,铁(氢)氧化物表面带正电荷,可以通过静电吸附带负电的磷酸阴离子,或者可进一步通过配位交换固定DIP[51],随着pH值的升高,碱性条件下羟基的配位竞争也抑制了DIP的吸附. 当pH<7.5时,DIP以单质子化的内圈络合物与铁结合,而当pH≥7.5时,DIP以非质子化的双齿双核形式与铁结合,而前者的结合方式使得DIP的吸附量远远大于后者[69]. 此外,酸性条件下弱结晶态的铁(氢)氧化物直接溶解也会导致磷的释放[18]. 同样,铁和DIP的共沉淀在pH值为5~9范围内都会发生,且Fe(Ⅲ)和DIP的共沉淀在pH值较低时更有利于形成磷酸铁沉淀(FePO4),而Fe(Ⅱ)与DIP的共沉淀在pH值为7~8之间时更有利于形成磷酸亚铁沉淀(Fe3(PO4)2),原因与不同pH值下FePO4和Fe(OH)3或Fe3(PO4)2和Fe(OH)2之间的竞争沉淀有关[54, 70]. 铁(氢)氧化物的比表面积、孔隙结构等也会影响其对DIP的吸附能力. 通过水解沉淀新形成的铁(氢)氧化物具有较大的比表面积,能够吸附更多的DIP,如Mao等研究发现新形成的水合氢氧化铁的比表面积为(1189±53)m2/g,远大于其他铁(氢)氧化物如Fe3O4(27.48 m2/g)、α-Fe2O3(106.9 m2/g)以及老化24 h后的水合氢氧化铁((476±30)m2/g)[71-73]. 弱结晶态的铁(氢)氧化物老化过程中比表面积逐渐降低,结构趋于稳定,但其对DIP的吸附能力也逐渐降低[74-75].
3 溶解性有机质对铁(氢)氧化物性质的影响DOM与铁的络合作用抑制了铁的水解沉淀,减小了水环境中铁相粒径,并通过促使矿物中铁的释放,增强了铁在水体中的溶解度和迁移转化能力. 天然水体中大约90 % 的溶解态铁与腐殖质结合,使得溶解态铁的浓度比用溶度积常数(Ksp(Fe(OH)3))计算所得大109~1010数量级[76]. DOM通过与Fe(Ⅱ)络合抑制氧化环境下Fe(Ⅱ)向Fe(Ⅲ)转化[77],或直接与Fe(Ⅲ)络合,从而抑制了上覆水体中铁的水解沉淀,且碳铁比越大,铁的水解沉淀率越小[78]. 同时,DOM还会影响水体中铁相的粒径,与无DOM存在时相比,DOM显著降低水体中铁相的粒径[77]. 除了直接与铁络合增加其溶解度外,DOM还可导致弱结晶态铁(氢)氧化物中铁的溶解释放:Wu等研究表明含更多含氧基团的低分子量(< 1000 Da)有机组分通过有机配体的机制促使铁的溶解[79].
DOM的存在会影响铁矿物结构组成的转化. 在铁和DOM共沉淀过程中,铁的形态以水铁矿为主,所占比例与碳铁比的值有关[31]. 研究表明,无论是通过吸附还是共沉淀,随着固定DOM的增多,水铁矿被Fe(Ⅱ)催化向高结晶度矿物的转变过程受到的抑制程度增大,且DOM类型会强烈地影响二次矿物的产生:从土壤有机层中提取的DOM会抑制水铁矿向针铁矿的转化,但会促进纤铁矿的形成,而作为多糖的聚半乳糖醛酸只使得水铁矿向针铁矿转化,无纤铁矿产生[38]. 此时DOM的作用一方面在于其在水铁矿上的吸附降低了水铁矿的比表面积,减少了水铁矿对Fe(Ⅱ)的吸附与接触,另一方面也与DOM与Fe(Ⅱ)的络合有关,从而减弱了Fe(Ⅱ)的催化作用. 同时,类似的研究表明即使没有Fe(Ⅱ)参与催化,DOM也会通过阻碍溶解点位或者抑制更稳定矿物的成核过程来抑制水铁矿向更稳定、结晶程度高的矿物转变[38, 80-82].
DOM与铁通过吸附或共沉淀形成Fe-DOM复合物会影响还原环境下铁的还原过程,影响铁的存在形式. Adhikari等研究表明Fe-DOM在微生物还原过程中,铁的还原速率与Fe-DOM复合物中的碳铁比有关:与纯水铁矿对比,低碳铁比(< 1.8)会抑制Fe-DOM复合物中铁的还原,而高碳铁比(>3.7)会促进Fe-DOM复合物中铁的还原释放[82]. 然而,Eusterhues等研究了基于吸附和共沉淀的Fe-DOM复合物在微生物还原和化学还原过程中铁的还原释放速率(碳/铁 < 3),发现随着矿物结合DOM的增多,铁的还原速率降低,且在相同的DOM负载量下,共沉淀形成的复合物比吸附形成的复合物活性更强,铁更容易被还原释放[83]. 造成上述不同结果的原因在于DOM组成结构及其与铁(氢)氧化物作用方式的复杂性. 基于“包裹”作用,有机碳附着在铁矿物表面,对内部起到保护作用,因此可减缓铁的还原. 然而DOM与铁共沉淀时形成的复合物晶体尺寸小、结晶度弱且具有较高的比表面积,因此可能更容易导致铁的还原. 众多研究还表明DOM具有良好的传递电子的能力,即“电子穿梭”,可以协助微生物还原过程中的电子转移进而加速铁的还原,且不同的有机组分所起到的传递电子的能力也不同[84-85]. 另外,有机质与铁结合还可以抑制铁(氢)氧化物向更加稳定的、低比表面积的、高结晶度的铁相转化(老化过程)[82],从而又使得铁(氢)氧化物易被还原物质还原. 总之,DOM对铁(氢)氧化物的影响与有机质含量、组成及其与铁的结合方式有关. 湖泊等内陆水体中的DOM往往具有不同的来源(如陆源和内源),不同水生生态系统类型中的DOM组成和浓度也不尽相同[4, 86],开展这些异质性的DOM与铁的相互作用对理解不同水生生态系统中铁的循环及其与铁紧密相关的污染物的迁移转化至关重要.
4 溶解性有机质对铁(氢)氧化物固定溶解性无机磷的影响铁(氢)氧化物对DIP的固定除了受上述如氧化还原环境、pH等因素的影响外,还受水体共存的DOM的影响:DOM一方面可以通过吸附或共沉淀过程抑制铁(氢)氧化物对DIP的吸附固定,另一方面也可能通过改变铁(氢)氧化物的组成结构进而促进DIP的吸附固定.
吸附过程中,DOM对铁(氢)氧化物固定DIP的抑制作用与DOM的分子大小、官能团的组成等密切相关. 基于DOM分子大小,Weng等发现小分子富里酸(FA:DOM的组成部分,酸碱皆溶,分子量较小)吸附在针铁矿表面时,会显著抑制针铁矿对DIP的吸附,而虽然腐殖酸(HA:DOM的组成部分,不溶于酸(pH < 2),分子量较大)与针铁矿的结合力很强,但其对针铁矿吸附DIP的影响不大,原因在于小分子的FA其空间位置更靠近铁氧化物表面,FA与DIP之间的静电排斥作用比HA强得多[20]. 此外,DOM的羧基密度、羧基质子化常数等也是影响针铁矿吸附含DIP在内的含氧阴离子的关键因素[87]. DOM中的羧基与金属矿物表面的羟基通过配位交换而结合,从而占据吸附点位[23, 87],抑制了DIP的吸附固定. 相较于亲水性的有机组分,矿物对疏水性有机物的吸附能力更强[88],因此疏水性DOM组分对DIP的竞争作用可能更为强烈.
共沉淀过程中,铁的沉淀是DIP被埋藏固定的先决条件,DOM能通过抑制共沉淀或者促进铁(氢)氧化物的溶解等抑制DIP的吸附固定. DOM富含的羧基、酚羟基可以通过与铁离子络合,抑制金属离子的水解沉淀过程进而影响DIP的去除,且不同DOM由于组分、结构差异,对金属离子水解沉淀的影响也不同. 酚类和羧基组分是影响金属离子水解沉淀的重要因素,基于共沉淀过程,HA含有的更多的类腐殖酸组分和含有酚和羧基的发色团等可以通过与金属离子络合而限制金属离子如Fe(Ⅲ)等的水解沉淀,进而抑制了DIP的共沉淀[89]. 因此,不同于吸附过程中基于分子大小机制的HA对DIP固定的较弱的影响,HA可通过共沉淀途径抑制铁对DIP的固定. 基于吸附过程,DOM通过静电吸附、配体交换等机制占据铁(氢)氧化物表面的吸附点位,抑制了DIP的吸附固定,DOM的分子量大小、官能团含量及组成等是关键影响因素; 基于共沉淀过程,可以与铁离子络合抑制其水解沉淀的官能团是抑制DIP固定的关键因素.
另一方面,有研究表明DOM还可促进铁(氢)氧化物对DIP的固定. 当HA的浓度较低时(< 0.5 mg/L),HA可以缓慢地促使菱铁矿中铁的还原释放,释放的铁离子遇氧化环境水解沉淀形成无定型的水铁矿,从而为DIP提供更多的吸附位点,促进金属矿物对DIP的吸附[90]. 与此类似的是,Chen等也发现,随着水铁矿吸附或者共沉淀DOM的增多,其向更加稳定和结晶度高的纤铁矿或针铁矿的转变(老化过程)受到抑制[38],而水铁矿具有较大的比表面积,从而能吸附固定更多的DIP.
5 溶解性无机磷对铁(氢)氧化物固定分馏溶解性有机质的影响DIP与铁(氢)氧化物的亲和力较强,会影响铁(氢)氧化物对DOM的吸附、共沉淀等过程. 一方面,DIP会抑制Fe(Ⅲ)的水解,并会改变铁的形态分布,DIP不同程度参与Fe(Ⅲ)的络合反应,在铁原子之间架桥,形成多核络合物,以Fe-OH-Fe和Fe-PO4-Fe结构共同控制着Fe(Ⅲ)的水解-聚合-沉淀反应[22],铁形态变化必然会影响其和DOM的结合. 另一方面,由于DIP在矿物上的吸附过程迅速(<24 h)[91],DIP对DOM在金属、黏土矿物上的竞争吸附对环境中DOM的吸附能产生快速而重大的影响. DIP的存在通过竞争吸附抑制矿物对DOM的吸附,且由于不同DOM组分的吸附能力不同,会导致被吸附DOM的非均质释放,进而影响DOM在固液界面的分馏. Luo等研究了DIP对HA在针铁矿上吸附分馏的影响,结果发现DIP可抑制并取代被吸附的陆源类腐殖酸组分,从而使得溶液中芳香性增加、DOM分子量变大[21]. Li等同样发现DIP的竞争吸附抑制了水铁矿对FA中芳香性组分和羧基的吸附,由此造成的DOM的分馏改变了水溶液中DOM与铜离子的结合能力[25]. 由于疏水性有机物更易被矿物吸附,DIP使得矿物对亲水性有机组分的吸附量显著下降[88].
DIP通过参与铁(氢)氧化物对DOM的吸附和共沉淀对DOM的固定和分馏造成影响,可改变水环境中的DOM组成与浓度,进而改变碳源的生物可利用性[92-93],并影响到与DOM相关的一些金属离子的迁移转化[94-95],从而对水生生态系统产生重要影响. 目前相关研究更多的是关注于DOM对DIP吸附固定的影响,两者之间相互作用及反馈机制对湖泊等水生态系统的影响目前还缺乏系统的研究,需进一步加强.
6 对湖泊内源磷释放、碳埋藏的可能影响根据以上文献梳理,本研究概括了在水生生态系统中,铁(氢)氧化物介导(基于吸附和共沉淀过程)的DOM和DIP的相互作用(图 2). 这些作用过程会对湖泊中磷、碳的存在形式产生影响.
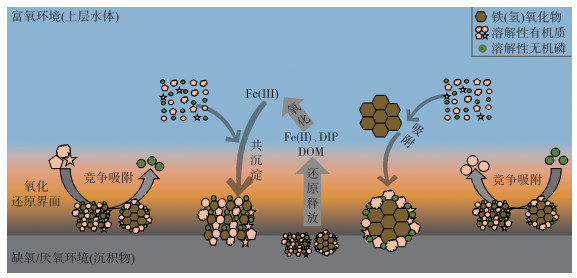
|
图 2 水生生态系统中铁(氢)氧化物介导(基于吸附和共沉淀过程)的DOM和DIP的相互作用示意图(不同浅橙色形状代表DOM的不同组分,其中圆形代表不易吸附的组分,其他形状代表容易吸附的组分) Fig.2 Schematic diagram on the interaction of dissolved organic matter and phosphate mediated by iron (hydr)oxides via adsorption and coprecipitation processes (Different light orange shapes represent different components of DOM, in which circle represents components that are not easy to adsorb, and other shapes represent components that are preferentially adsorbed) |
湖泊生态系统中的DOM来源多样,组成复杂,在浓度、分子大小、组成上均存在差异,其对铁(氢)氧化物固定DIP的影响也可能不同. 富营养化湖泊往往含有较高浓度的DOC [96],此时高浓度的DOC可能作用于沉积物或间隙水中的铁相,置换DIP或者促进铁的溶解释放[28, 97-99],进而导致沉积物或间隙水中内源磷的释放,对湖泊富营养化产生正反馈[100]. 浅水湖泊的两种典型状态——草、藻型湖泊中,其DOM组成存在差异. 研究表明藻型湖泊生态系统中的DOM含有更多的类蛋白物质,而草型湖泊生态系统中的DOM含有更多的类腐殖酸和富里酸物质[101],针铁矿优先吸附类蛋白组分,从而导致对藻源DOM的吸附能力强于草源DOM [102]. 因此,相较于草源DOM,藻源DOM更可能通过占据吸附点位抑制铁(氢)氧化物对DIP的固定. 除吸附作用外,张巧颖等对比研究了草、藻源DOM对Fe(Ⅲ)和DIP共沉淀的影响,发现DOM的存在会显著降低Fe(Ⅲ)的水解沉淀,进而抑制DIP的固定,且藻源DOM抑制Fe(Ⅲ)的水解沉淀作用更强烈,从而导致较少的DIP被固定[78]. 因此,对于藻型湖泊生态系统或者富营养化湖泊,藻源DOM一方面通过强吸附抑制铁(氢)氧化物对DIP的吸附; 另一方面通过强抑制Fe(Ⅲ)的水解,减弱铁和DIP的共沉淀. 这可能是维持富营养化湖泊较高磷浓度的一个重要原因.
一方面,铁(氢)氧化物通过吸附或者共沉淀固定DOM使得有机碳埋藏在沉积物中,减缓碳作为温室气体释放到大气中[1, 30],直接阻碍碳的循环. 另一方面,铁(氢)氧化物对有机碳的埋藏往往伴随着DOM的分馏,使得水体中DOM组分结构和性质发生变化,影响其生物可利用性,进而影响碳的循环过程. 如有研究表明矿物对DOM的吸附分馏导致小分子DOM保留在水中,而小分子DOM通常生物活性较高[92-93]. 类蛋白和脂肪族化合物容易在微生物的新陈代谢过程中被降解[93],而矿物对类蛋白组分的优先吸附使其被固定在沉积物中[101-102],进而可能抑制了有机碳的矿化过程. 不同DOM组分对温室气体CO2、CH4和N2O产生的影响也不同[103-104],如Amaral等研究发现河口区CO2的产生与陆地和微生物来源的类腐殖酸组分有关,尤其是芳香性组分,CH4的产生与较多的有机组分相关,而N2O的产生与人类活动产生的小分子量高氮含量的类蛋白组分有关[104]. 此外DIP也可影响DOM的固定分馏,在引起湖泊富营养化的同时改变温室气体的排放特征. 如Sun等研究发现富营养化湖泊抑制了CO2的排放,但促进了CH4的排放[105],这可能与矿物对芳香性组分的固定埋藏有关. 因此,铁(氢)氧化物及DIP对DOM的固定分馏一方面直接埋藏部分有机碳; 另一方面又通过改变水环境中有机质的组成和其生物可利用性改变了有机碳的矿化过程,进而对碳的埋藏产生影响. 但不同环境条件下DOM的分馏对水中有机碳矿化是促进还是抑制,仍需要进一步的研究.
7 结论与展望铁(氢)氧化物介导的DOM和DIP的固定是生态系统中碳、磷的生物地球化学循环的重要环节. 铁(氢)氧化物通过吸附和共沉淀过程优先固定芳香性较强、类蛋白及富含羧基的化合物等,造成DOM的分馏,改变了水环境中DOM的组成和结构. DOM和DIP都可通过竞争吸附、抑制铁的水解沉淀等途径影响彼此的固定或分馏. 同时,DOM的组成结构及含量会影响DOM—Fe—DIP复合物的稳定性,使其在还原环境下表现出不同的释放特征,改变碳、磷的埋藏过程.
DOM复杂的组成和结构及其与铁、DIP的多种作用方式直接或间接地影响着湖泊等生态系统中的碳、磷循环过程. 尽管已有相关研究关注于铁(氢)氧化物对DOM的分馏、DOM对铁(氢)氧化物结构和DIP固定的影响等,但系统地研究三者之间相互作用、反馈机制及其对生态系统的影响还比较匮乏,尤其是对水生态系统而言. 湖泊等水生态系统接收内外源、自然和人为产生的DOM,不同的水生态系统其DOM组成与结构差异巨大,这些不同来源与组成的DOM在特定的环境下如何与铁、DIP相互作用,进而影响系统中碳、铁、磷的迁移转化和埋藏过程,以及对水生态系统可能产生的影响如富营养化等,都需要进一步深入研究.
综上,我们建议开展以下几方面的研究:
1) 基于室内、原位实验和监测,探究不同来源DOM(富营养化产生的大量内源DOM、陆地系统产生的外源DOM、人类活动输入的外源DOM(如生活污水DOM))与Fe(离子)氧化物结合的差异以及对DIP的影响,明确不同来源和结构DOM输入对湖泊富营养化进程的影响.
2) 不同环境条件下DOM—Fe—DIP三者相互作用的差异及形成的三元复合物的稳定性和生物有效性. 如不同C/Fe/P比值下形成的复合物在氧化还原环境、pH、温度等条件发生变化时,复合物氧化还原、溶解以及金属、碳和磷的释放潜力等; 揭示DOM存在对铁结合态磷释放的影响.
3) DIP对铁(氢)氧化物固定、分馏DOM的影响,及其分馏后DOM的生物可利用性,有助于全面了解湖泊中DOM的迁移转化及其对营养盐输入的响应.
| [1] |
Lalonde K, Mucci A, Ouellet A et al. Preservation of organic matter in sediments promoted by iron. Nature, 2012, 483(7388): 198-200. DOI:10.1038/nature10855 |
| [2] |
Zhang YL, Zhou L, Zhou YQ et al. Chromophoric dissolved organic matter in inland waters: Present knowledge and future challenges. Science of the Total Environment, 2021, 759: 143550. DOI:10.1016/j.scitotenv.2020.143550 |
| [3] |
Wei ZM, Zhang X, Wei YQ et al. Fractions and biodegradability of dissolved organic matter derived from different composts. Bioresource Technology, 2014, 161: 179-185. DOI:10.1016/j.biortech.2014.03.032 |
| [4] |
Song KS, Wen ZD, Shang YX et al. Quantification of dissolved organic carbon (DOC) storage in lakes and reservoirs of mainland China. Journal of Environmental Management, 2018, 217: 391-402. DOI:10.1016/j.jenvman.2018.03.121 |
| [5] |
Tranvik LJ, Downing JA, Cotner JB et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnology and Oceanography, 2009, 54(6): 2298-2314. DOI:10.4319/lo.2009.54.6_part_2.2298 |
| [6] |
Pang JL, Xu YH, He YX et al. Molecular characteristics of surface dissolved organic matter in Meiliang Bay of Lake Taihu over the algal blooming-disappearance cycle. J Lake Sci, 2020, 32(6): 1599-1609. [庞佳丽, 许燕红, 何毓新等. 太湖梅梁湾藻华暴发消退周期表层水体溶解性有机质分子特征. 湖泊科学, 2020, 32(6): 1599-1609. DOI:10.18307/2020.0603] |
| [7] |
Schindler DW, Hecky RE, Findlay DL et al. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(32): 11254-11258. DOI:10.1073/pnas.0805108105 |
| [8] |
Fan CX, Yang LY, Zhang L. The vertical distributions of nitrogen and phosphorus in the sediment and interstitial water in Taihu Lake and their interrelations. J Lake Sci, 2000, 12(4): 359-366. [范成新, 杨龙元, 张路. 太湖底泥及其间隙水中氮磷垂直分布及相互关系分析. 湖泊科学, 2000, 12(4): 359-366. DOI:10.18307/2000.0410] |
| [9] |
Søndergaard M, Jensen JP, Jeppesen E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia, 2003, 506/507/508/509(1/2/3): 135-145. DOI:10.1023/b:hydr.0000008611.12704.dd |
| [10] |
Jeppesen E, Søndergaard M, Jensen JP et al. Lake responses to reduced nutrient loading—an analysis of contemporary long-term data from 35 case studies. Freshwater Biology, 2005, 50(10): 1747-1771. DOI:10.1111/j.1365-2427.2005.01415.x |
| [11] |
Cavalcante H, Araújo F, Noyma NP et al. Phosphorus fractionation in sediments of tropical semiarid reservoirs. Science of the Total Environment, 2018, 619/620: 1022-1029. DOI:10.1016/j.scitotenv.2017.11.204 |
| [12] |
Chen MS, Ding SM, Wu YX et al. Phosphorus mobilization in lake sediments: Experimental evidence of strong control by iron and negligible influences of manganese redox reactions. Environmental Pollution, 2019, 246: 472-481. DOI:10.1016/j.envpol.2018.12.031 |
| [13] |
Jin XC, Wang SR, Pang Y et al. Phosphorus fractions and the effect of pH on the phosphorus release of the sediments from different trophic areas in Taihu Lake, China. Environmental Pollution, 2006, 139(2): 288-295. DOI:10.1016/j.envpol.2005.05.010 |
| [14] |
Wang S, Dou H. Chinese lakes. Beijing: Science Press, 1998. [王苏民, 窦鸿身. 中国湖泊志. 北京: 科学出版社, 1998.]
|
| [15] |
Gong MD, Jin ZF, Wang Y et al. Coupling between iron and phosphorus in sediments of shallow lakes in the middle and lower reaches of Yangtze River using diffusive gradients in thin films (DGT). J Lake Sci, 2017, 29(5): 1103-1111. [龚梦丹, 金增锋, 王燕等. 长江中下游典型浅水湖泊沉积物水界面磷与铁的耦合关系. 湖泊科学, 2017, 29(5): 1103-1111. DOI:10.18307/2017.0508] |
| [16] |
Poulin BA, Ryan JN, Aiken GR. Effects of iron on optical properties of dissolved organic matter. Environmental Science & Technology, 2014, 48(17): 10098-10106. DOI:10.1021/es502670r |
| [17] |
Shi ZL, Li F, Yao SH. Effect of small organic acid anions on the adsorption of phosphate anions onto synthetic goethite from aqueous solution. Adsorption Science & Technology, 2010, 28(10): 885-893. DOI:10.1260/0263-6174.28.10.885 |
| [18] |
Johnson SE, Loeppert RH. Role of organic acids in phosphate mobilization from iron oxide. Soil Science Society of America Journal, 2006, 70(1): 222-234. DOI:10.2136/sssaj2005.0012 |
| [19] |
Hiemstra T, Mia S, Duhaut PB et al. Natural and pyrogenic humic acids at goethite and natural oxide surfaces interacting with phosphate. Environmental Science & Technology, 2013, 47(16): 9182-9189. DOI:10.1021/es400997n |
| [20] |
Weng LP, vanRiemsdijk WH, Hiemstra T. Humic nanoparticles at the oxide-water interface: Interactions with phosphate ion adsorption. Environmental Science & Technology, 2008, 42(23): 8747-8752. DOI:10.1021/es801631d |
| [21] |
Luo CY, Wen SL, An SL et al. Phosphate alters the compositional characteristics of humic acid adsorbed onto goethite. Journal of Soils and Sediments, 2021, 21(10): 3352-3366. DOI:10.1007/s11368-021-02973-4 |
| [22] |
Tian BZ, Tang HX. The hydrolyzed and other chemical characters of ferric chloride solution containing phosphate. Environmental Chemistry, 1995, 14(4): 329-337. [田宝珍, 汤鸿霄. 含磷酸盐的三氯化铁水解溶液的化学特征. 环境化学, 1995, 14(4): 329-337.] |
| [23] |
Groeneveld M, Catalán N, Attermeyer K et al. Selective adsorption of terrestrial dissolved organic matter to inorganic surfaces along a boreal inland water continuum. Journal of Geophysical Research: Biogeosciences, 2020, 125(3): e2019JG005236. DOI:10.1029/2019JG005236 |
| [24] |
Sowers TD, Holden KL, Coward EK et al. Dissolved organic matter sorption and molecular fractionation by naturally occurring bacteriogenic iron (oxyhydr)oxides. Environmental Science & Technology, 2019, 53(8): 4295-4304. DOI:10.1021/acs.est.9b00540 |
| [25] |
Li JH, Ding Y, Shi ZQ. Binding properties of fulvic acid before and after fractionation on ferrihydrite: Effects of phosphate. ACS Earth and Space Chemistry, 2021, 5(6): 1535-1543. DOI:10.1021/acsearthspacechem.1c00069 |
| [26] |
Chassé AW, Ohno T. Higher molecular mass organic matter molecules compete with orthophosphate for adsorption to iron (oxy)hydroxide. Environmental Science & Technology, 2016, 50(14): 7461-7469. DOI:10.1021/acs.est.6b01582 |
| [27] |
Chen CM, Kukkadapu R, Sparks DL. Influence of coprecipitated organic matter on Fe(aq)2+-catalyzed transformation of ferrihydrite: Implications for carbon dynamics. Environmental Science & Technology, 2015, 49(18): 10927-10936. DOI:10.1021/acs.est.5b02448 |
| [28] |
Wu WL, Yan JL, Jiang T et al. Natural organic matter-metal ion/oxide-phosphorus complexes in environment: A review. Journal of Ecology and Rural Environment, 2019, 35(9): 1089-1096. [吴文丽, 闫金龙, 江韬等. 天然有机质-金属离子/氧化物-磷复合物的研究进展. 生态与农村环境学报, 2019, 35(9): 1089-1096.] |
| [29] |
Kleber M, Bourg IC, Coward EK et al. Dynamic interactions at the mineral-organic matter interface. Nature Reviews Earth & Environment, 2021, 2(6): 402-421. DOI:10.1038/s43017-021-00162-y |
| [30] |
Chen CM, Dynes JJ, Wang J et al. Properties of Fe-organic matter associations via coprecipitation versus adsorption. Environmental Science & Technology, 2014, 48(23): 13751-13759. DOI:10.1021/es503669u |
| [31] |
Han LF, Yang Y, Sun K. Co-precipitation triggered molecular fractionation of dissolved organic matter at the ferrihydrite/water interface. Acta Pedologica Sinica, 2022, 59(3): 767-775. [韩兰芳, 杨妍, 孙可. 共沉淀引发的溶解性有机质在水铁矿/水界面的分子分馏特性. 土壤学报, 2022, 59(3): 767-775. DOI:10.11766/trxb202009030393HAN] |
| [32] |
Du HH, Peacock CL, Chen WL et al. Binding of Cd by ferrihydrite organo-mineral composites: Implications for Cd mobility and fate in natural and contaminated environments. Chemosphere, 2018, 207: 404-412. DOI:10.1016/j.chemosphere.2018.05.092 |
| [33] |
Sollins P, Swanston C, Kleber M et al. Organic C and N stabilization in a forest soil: Evidence from sequential density fractionation. Soil Biology and Biochemistry, 2006, 38(11): 3313-3324. DOI:10.1016/j.soilbio.2006.04.014 |
| [34] |
Kaiser K, Guggenberger G. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils. Organic Geochemistry, 2000, 31(7/8): 711-725. DOI:10.1016/S0146-6380(00)00046-2 |
| [35] |
Hunter WR, Niederdorfer R, Gernand A et al. Metabolism of mineral-sorbed organic matter and microbial lifestyles in fluvial ecosystems. Geophysical Research Letters, 2016, 43(4): 1582-1588. DOI:10.1002/2016GL067719 |
| [36] |
Ritter K, Aiken GR, Ranville JF et al. Evidence for the aquatic binding of arsenate by natural organic matter-suspended Fe(Ⅲ). Environmental Science & Technology, 2006, 40(17): 5380-5387. DOI:10.1021/es0519334 |
| [37] |
Sharma P, Ofner J, Kappler A. Formation of binary and ternary colloids and dissolved complexes of organic matter, Fe and As. Environmental Science & Technology, 2010, 44(12): 4479-4485. DOI:10.1021/es100066s |
| [38] |
Chen CM, Sparks DL. Fe(Ⅱ)-induced mineral transformation of ferrihydrite-organic matter adsorption and co-precipitation complexes in the absence and presence of As(Ⅲ). ACS Earth and Space Chemistry, 2018, 2(11): 1095-1101. DOI:10.1021/acsearthspacechem.8b00041 |
| [39] |
Shen YH. Sorption of natural dissolved organic matter on soil. Chemosphere, 1999, 38(7): 1505-1515. DOI:10.1016/S0045-6535(98)00371-3 |
| [40] |
Grybos M, Davranche M, Gruau G et al. Increasing pH drives organic matter solubilization from wetland soils under reducing conditions. Geoderma, 2009, 154(1/2): 13-19. DOI:10.1016/j.geoderma.2009.09.001 |
| [41] |
Gu B, Schmitt J, Chen Z et al. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. Environmental Science & Technology, 1994, 28(1): 38-46. DOI:10.1021/es00050a007 |
| [42] |
Lee BM, Seo YS, Hur J. Investigation of adsorptive fractionation of humic acid on graphene oxide using fluorescence EEM-PARAFAC. Water Research, 2015, 73: 242-251. DOI:10.1016/j.watres.2015.01.020 |
| [43] |
Riedel T, Zak D, Biester H et al. Iron traps terrestrially derived dissolved organic matter at redox interfaces. PNAS, 2013, 110(25): 10101-10105. DOI:10.1073/pnas.1221487110 |
| [44] |
Chen CM, Hall SJ, Coward E et al. Iron-mediated organic matter decomposition in humid soils can counteract protection. Nature Communications, 2020, 11: 2255. DOI:10.1038/s41467-020-16071-5 |
| [45] |
Wagai R, Mayer LM. Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochimica et Cosmochimica Acta, 2007, 71(1): 25-35. DOI:10.1016/j.gca.2006.08.047 |
| [46] |
Coward EK, Ohno T, Sparks DL. Direct evidence for temporal molecular fractionation of dissolved organic matter at the ironoxyhydroxide interface. Environmental Science & Technology, 2019, 53(2): 642-650. DOI:10.1021/acs.est.8b04687 |
| [47] |
Du YX, Ramirez CE, Jaffé R. Fractionation of dissolved organic matter by co-precipitation with iron: Effects of composition. Environmental Processes, 2018, 5(1): 5-21. DOI:10.1007/s40710-017-0281-4 |
| [48] |
Riedel T, Biester H, Dittmar T. Molecular fractionation of dissolved organic matter with metal salts. Environmental Science & Technology, 2012, 46(8): 4419-4426. DOI:10.1021/es203901u |
| [49] |
Han LF, Sun K, Keiluweit M et al. Mobilization of ferrihydrite-associated organic carbon during Fe reduction: Adsorption versus coprecipitation. Chemical Geology, 2019, 503: 61-68. DOI:10.1016/j.chemgeo.2018.10.028 |
| [50] |
Oren A, Chefetz B. Sorptive and desorptive fractionation of dissolved organic matter by mineral soil matrices. Journal of Environmental Quality, 2012, 41(2): 526-533. DOI:10.2134/jeq2011.0362 |
| [51] |
Wang QP, Liao ZY, Yao DX et al. Phosphorus immobilization in water and sediment using iron-based materials: A review. Science of the Total Environment, 2021, 767: 144246. DOI:10.1016/j.scitotenv.2020.144246 |
| [52] |
Weng YT, Vekeman J, Zhang HL et al. Unravelling phosphate adsorption on hydrous ferric oxide surfaces at the molecular level. Chemosphere, 2020, 261: 127776. DOI:10.1016/j.chemosphere.2020.127776 |
| [53] |
Elzinga EJ, Sparks DL. Phosphate adsorption onto hematite: An in situ ATR-FTIR investigation of the effects of pH and loading level on the mode of phosphate surface complexation. Journal of Colloid and Interface Science, 2007, 308(1): 53-70. DOI:10.1016/j.jcis.2006.12.061 |
| [54] |
Zhang M, Zheng K, Jin JJ et al. Effects of Fe(Ⅱ)/P ratio and pH on phosphorus removal by ferrous salt and approach to mechanisms. Separation and Purification Technology, 2013, 118: 801-805. DOI:10.1016/j.seppur.2013.08.034 |
| [55] |
Wen SL, Wang HW, Wu T et al. Vertical profiles of phosphorus fractions in the sediment in a chain of reservoirs in North China: Implications for pollution source, bioavailability, and eutrophication. Science of the Total Environment, 2020, 704: 135318. DOI:10.1016/j.scitotenv.2019.135318 |
| [56] |
Liu C, Shao SG, Shen QS et al. Effects of riverine suspended particulate matter on the post-dredging increase in internal phosphorus loading across the sediment-water interface. Environmental Pollution, 2016, 211: 165-172. DOI:10.1016/j.envpol.2015.12.045 |
| [57] |
Rydin E. Potentially mobile phosphorus in Lake Erken sediment. Water Research, 2000, 34(7): 2037-2042. DOI:10.1016/S0043-1354(99)00375-9 |
| [58] |
Yuan HZ, Shen J, Liu EF et al. Characteristic of phosphorus release with the control of pH of sediments from Meiliang Bay, Lake Taihu. J Lake Sci, 2009, 21(5): 663-668. [袁和忠, 沈吉, 刘恩峰等. 模拟水体pH控制条件下太湖梅梁湾沉积物中磷的释放特征. 湖泊科学, 2009, 21(5): 663-668. DOI:10.18307/2009.0508] |
| [59] |
Li DP, Huang Y. Sedimentary phosphorus fractions and bioavailability as influenced by repeated sediment resuspension. Ecological Engineering, 2010, 36(7): 958-962. DOI:10.1016/j.ecoleng.2010.04.014 |
| [60] |
Yang CH, Yang P, Geng J et al. Sediment internal nutrient loading in the most polluted area of a shallow eutrophic lake (Lake Chaohu, China) and its contribution to lake eutrophication. Environmental Pollution, 2020, 262: 114292. DOI:10.1016/j.envpol.2020.114292 |
| [61] |
Wang C, Zou LM, Wang PF et al. Distribution and correlation of P and Fe fractions in sediments of typical urban shallow lakes. Environmental Science, 2008, 29(12): 3400-3404. [王超, 邹丽敏, 王沛芳等. 典型城市浅水湖泊沉积物中磷与铁的形态分布及相关关系. 环境科学, 2008, 29(12): 3400-3404. DOI:10.13227/j.hjkx.2008.12.041] |
| [62] |
Zhang SJ, Qi QJ, Wang SR et al. Effects of organic matter, manganese and iron on phosphorus fractions and release in the sediments of Erhai Lake. Research of Environmental Sciences, 2011, 24(4): 371-377. [张仕军, 齐庆杰, 王圣瑞等. 洱海沉积物有机质、铁、锰对磷的赋存特征和释放影响. 环境科学研究, 2011, 24(4): 371-377. DOI:10.13198/j.res.2011.04.11.zhangshj.006] |
| [63] |
Wang Q, Jiang X, Jin Xc et al. Distribution of phosphorus fractions and bio-available phosphorus forms and their relationship in the sediments from different regions of Lake Taihu. J Lake Sci, 2006, 18(2): 120-126. [王琦, 姜霞, 金相灿等. 太湖不同营养水平湖区沉积物磷形态与生物可利用磷的分布及相互关系. 湖泊科学, 2006, 18(2): 120-126. DOI:10.18307/2006.0204] |
| [64] |
Amirbahman A, Lake BA, Norton SA. Seasonal phosphorus dynamics in the surficial sediment of two shallow temperate lakes: A solid-phase and pore-water study. Hydrobiologia, 2013, 701(1): 65-77. DOI:10.1007/s10750-012-1257-z |
| [65] |
Lin J, Su Y, Han C et al. In situ and combined variations of phosphorus, iron and sulfur across the sediment-water interface of Lake Luoma. China Environmental Science, 2021, 41(12): 5637-5645. [林建宇, 苏雅玲, 韩超等. 骆马湖泥-水界面磷铁硫原位同步变化特征. 中国环境科学, 2021, 41(12): 5637-5645. DOI:10.3969/j.issn.1000-6923.2021.12.019] |
| [66] |
Ren J, Li N, Li L et al. Granulation and ferric oxides loading enable biochar derived from cotton stalk to remove phosphate from water. Bioresource Technology, 2015, 178: 119-125. DOI:10.1016/j.biortech.2014.09.071 |
| [67] |
Guo ZL, Li L, Liu XY et al. Analysis of the inorganic phosphorus sorption characteristics of two kinds of iron oxides. Periodical of Ocean University of China, 2021, 51(8): 42-48. [郭智俐, 李苓, 刘晓月等. 两种铁氧化物对无机磷的吸附特征分析. 中国海洋大学学报: 自然科学版, 2021, 51(8): 42-48.] |
| [68] |
Jang M, Min SH, Kim TH et al. Removal of arsenite and arsenate using hydrous ferric oxide incorporated into naturally occurring porous diatomite. Environmental Science & Technology, 2006, 40(5): 1636-1643. DOI:10.1021/es051501t |
| [69] |
Arai Y, Sparks DL. ATR-FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite-water interface. Journal of Colloid and Interface Science, 2001, 241(2): 317-326. DOI:10.1006/jcis.2001.7773 |
| [70] |
Caravelli AH, Contreras EM, Zaritzky NE. Phosphorous removal in batch systems using ferric chloride in the presence of activated sludges. Journal of Hazardous Materials, 2010, 177(1/2/3): 199-208. DOI:10.1016/j.jhazmat.2009.12.018 |
| [71] |
Mao YP, Pham AN, Xin YJ et al. Effects of pH, floc age and organic compounds on the removal of phosphate by pre-polymerized hydrous ferric oxides. Separation and Purification Technology, 2012, 91: 38-45. DOI:10.1016/j.seppur.2011.09.045 |
| [72] |
Hao HT, Wang YL, Shi BY. NaLa(CO3)2 hybridized with Fe3O4 for efficient phosphate removal: Synthesis and adsorption mechanistic study. Water Research, 2019, 155: 1-11. DOI:10.1016/j.watres.2019.01.049 |
| [73] |
Liang HN, Liu K, Ni YH. Synthesis of mesoporous α-Fe2O3 using cellulose nanocrystals as template and its use for the removal of phosphate from wastewater. Journal of the Taiwan Institute of Chemical Engineers, 2017, 71: 474-479. DOI:10.1016/j.jtice.2016.12.008 |
| [74] |
Mao YP, Yue QY. Kinetic modeling of phosphate adsorption by preformed and in situ formed hydrous ferric oxides at circumneutral pH. Scientific Reports, 2016, 6: 35292. DOI:10.1038/srep35292 |
| [75] |
Conidi D, Parker WJ. The effect of solids residence time on phosphorus adsorption to hydrous ferric oxide floc. Water Research, 2015, 84: 323-332. DOI:10.1016/j.watres.2015.07.046 |
| [76] |
Fu P, Liu C, Wu F. Binding of metal-ions with humic substances in aquatic environments: A review. Chinese Journal of Ecology, 2004, 23(6): 143-148. [傅平青, 刘丛强, 吴丰昌. 水环境中腐殖质金属离子键合作用研究进展. 生态学杂志, 2004, 23(6): 143-148. DOI:10.13292/j.1000-4890.2004.0217] |
| [77] |
Gaffney JW, White KN, Boult S. Oxidation state and size of Fe controlled by organic matter in natural waters. Environmental Science & Technology, 2008, 42(10): 3575-3581. DOI:10.1021/es702880a |
| [78] |
Zhang QY. Co-precipitation of dissolved organic matter from the macrophyte-dominated zone/the algae-dominated zone and iron, phosphorus in the lake sediment[Dissertation]. Beijing: University of Chinese Academy of Sciences, 2020. [张巧颖. 草/藻湖区可溶性有机质和铁、磷的共沉淀作用研究[学位论文]. 北京: 中国科学院大学, 2020. ]
|
| [79] |
Wu H, Chen ZH, Sheng F et al. Mechanisms for the dissolved biochar promoted iron dissolution and consequential chromium release. Science of the Total Environment, 2021, 796: 148923. DOI:10.1016/j.scitotenv.2021.148923 |
| [80] |
Cornell RM. Influence of organic anions on the crystallization of ferrihydrite. Clays and Clay Minerals, 1979, 27(6): 402-410. DOI:10.1346/ccmn.1979.0270602 |
| [81] |
Xiao W, Jones AM, Li XM et al. Effect of Shewanella oneidensis on the kinetics of Fe(Ⅱ)-catalyzed transformation of ferrihydrite to crystalline iron oxides. Environmental Science & Technology, 2018, 52(1): 114-123. DOI:10.1021/acs.est.7b05098 |
| [82] |
Adhikari D, Zhao Q, Das K et al. Dynamics of ferrihydrite-bound organic carbon during microbial Fe reduction. Geochimica et Cosmochimica Acta, 2017, 212: 221-233. DOI:10.1016/j.gca.2017.06.017 |
| [83] |
Eusterhues K, Hädrich A, Neidhardt J et al. Reduction of ferrihydrite with adsorbed and coprecipitated organic matter: Microbial reduction by Geobacter bremensis vs. abiotic reduction by Na-dithionite. Biogeosciences, 2014, 11(18): 4953-4966. DOI:10.5194/bg-11-4953-2014 |
| [84] |
Lovley DR, Coates JD, Blunt-Harris EL et al. Humic substances as electron acceptors for microbial respiration. Nature, 1996, 382(6590): 445-448. DOI:10.1038/382445a0 |
| [85] |
Jiang J, Kappler A. Kinetics of microbial and chemical reduction of humic substances: Implications for electron shuttling. Environmental Science & Technology, 2008, 42(10): 3563-3569. DOI:10.1021/es7023803 |
| [86] |
Kothawala DN, Stedmon CA, Müller RA et al. Controls of dissolved organic matter quality: Evidence from a large-scale boreal lake survey. Global Change Biology, 2014, 20(4): 1101-1114. DOI:10.1111/gcb.12488 |
| [87] |
Deng YX, Weng LP, Li YT et al. Understanding major NOM properties controlling its interactions with phosphorus and arsenic at goethite-water interface. Water Research, 2019, 157: 372-380. DOI:10.1016/j.watres.2019.03.077 |
| [88] |
Kaiser K, Zech W. Competitive sorption of dissolved organic matter fractions to soils and related mineral phases. Soil Science Society of America Journal, 1997, 61(1): 64-69. DOI:10.2136/sssaj1997.03615995006100010011x |
| [89] |
Ding X, Li ZW, Xu WH et al. Restriction of dissolved organic matter on the stabilization of Cu(Ⅱ) by phosphate. Environmental Science and Pollution Research International, 2021, 28(18): 22902-22912. DOI:10.1007/s11356-021-12398-8 |
| [90] |
Xing BB, Ouyang MZ, Graham N et al. Enhancement of phosphate adsorption during mineral transformation of natural siderite induced by humic acid: Mechanism and application. Chemical Engineering Journal, 2020, 393: 124730. DOI:10.1016/j.cej.2020.124730 |
| [91] |
Li XD, Kuang Y, Chen JB et al. Competitive adsorption of phosphate and dissolved organic carbon on lanthanum modified zeolite. Journal of Colloid and Interface Science, 2020, 574: 197-206. DOI:10.1016/j.jcis.2020.04.050 |
| [92] |
Chen ML, Jaffé R. Photo-and bio-reactivity patterns of dissolved organic matter from biomass and soil leachates and surface waters in a subtropical wetland. Water Research, 2014, 61: 181-190. DOI:10.1016/j.watres.2014.03.075 |
| [93] |
Zhou L, Zhou YQ, Tang XM et al. Resource aromaticity affects bacterial community successions in response to different sources of dissolved organic matter. Water Research, 2021, 190: 116776. DOI:10.1016/j.watres.2020.116776 |
| [94] |
Chen MS, Ding SM, Li C et al. High cadmium pollution from sediments in a eutrophic lake caused by dissolved organic matter complexation and reduction of manganese oxide. Water Research, 2021, 190: 116711. DOI:10.1016/j.watres.2020.116711 |
| [95] |
Liu MX, Han XK, Liu CQ et al. Differences in the spectroscopic characteristics of wetland dissolved organic matter binding with Fe3+, Cu2+, Cd2+, Cr3+ and Zn2+. Science of the Total Environment, 2021, 800: 149476. DOI:10.1016/j.scitotenv.2021.149476 |
| [96] |
Thurman EM ed. Amount of organic carbon in natural waters. Organic Geochemistry of Natural Waters. Dordrecht: Springer Netherlands, 1985, 7-65. DOI:10.1007/978-94-009-5095-5_2 |
| [97] |
Geelhoed JS, van Riemsdijk WH, Findenegg GR. Simulation of the effect of citrate exudation from roots on the plant availability of phosphate adsorbed on goethite. European Journal of Soil Science, 1999, 50(3): 379-390. DOI:10.1046/j.1365-2389.1999.00251.x |
| [98] |
Kirk GJD, Santos EE, Santos MB. Phosphate solubilization by organic anion excretion from rice growing in aerobic soil: Rates of excretion and decomposition, effects on rhizosphere pH and effects on phosphate solubility and uptake. New Phytologist, 1999, 142(2): 185-200. DOI:10.1046/j.1469-8137.1999.00400.x |
| [99] |
Deng H, Bao L, Yang HY et al. Simulation study on release dynamics of various forms of phosphorus in sediments of Caohai, Dianchi Lake under decline of cyanobacteria. Wetland Science, 2019, 17(2): 249-254. [邓洪, 包立, 杨浩瑜等. 蓝藻衰亡下滇池草海沉积物中各形态磷释放动态的模拟研究. 湿地科学, 2019, 17(2): 249-254.] |
| [100] |
Xie FZ, Dai ZL, Zhu YR et al. Adsorption of phosphate by sediments in a eutrophic lake: Isotherms, kinetics, thermodynamics and the influence of dissolved organic matter. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 562: 16-25. DOI:10.1016/j.colsurfa.2018.11.009 |
| [101] |
Xu HC, Yu GH, Yang LY et al. Combination of two-dimensional correlation spectroscopy and parallel factor analysis to characterize the binding of heavy metals with DOM in lake sediments. Journal of Hazardous Materials, 2013, 263: 412-421. DOI:10.1016/j.jhazmat.2013.09.042 |
| [102] |
Zhang Q, Ju Q, Li JX et al. Heterogeneous adsorption of macrophyte-and algae-derived dissolved organic matter on goethite in freshwater lakes. J Lake Sci, 2020, 32(4): 1041-1049. [章奇, 居琪, 李健欣等. 针铁矿对湖泊草、藻来源可溶有机质的非均质吸附. 湖泊科学, 2020, 32(4): 1041-1049. DOI:10.18307/2020.0413] |
| [103] |
Chen H, Ye JF, Zhou YF et al. Variations in CH4 and CO2 productions and emissions driven by pollution sources in municipal sewers: An assessment of the role of dissolved organic matter components and microbiota. Environmental Pollution, 2020, 263: 114489. DOI:10.1016/j.envpol.2020.114489 |
| [104] |
Amaral V, Ortega T, Romera-Castillo C et al. Linkages between greenhouse gases (CO2, CH4, and N2O) and dissolved organic matter composition in a shallow estuary. Science of the Total Environment, 2021, 788: 147863. DOI:10.1016/j.scitotenv.2021.147863 |
| [105] |
Sun HY, Lu XX, Yu RH et al. Eutrophication decreased CO2 but increased CH4 emissions from lake: A case study of a shallow Lake Ulansuhai. Water Research, 2021, 201: 117363. DOI:10.1016/j.watres.2021.117363 |
 2022, Vol. 34
2022, Vol. 34 


