重建大陆古气候与古环境一直是地质学研究的热点问题之一,这对更好地了解地球历史,预测未来气候变化具有重要意义。温度作为气候变化的重要因子,其变化显著地影响着气候带的分布、动植物的生存、人类健康及农业生产等。因此,厘清温度的定量变化至关重要。然而,当前依靠仪器记录温度变化在时间和空间上存在限制,并且近年来温度还广泛受到人类活动的影响,还不足以理解自然背景下温度变化的规律及其变化机制。
微生物细胞膜脂的甘油二烷基甘油四醚(简称GDGTs)广泛存在于各种沉积环境中,包括海洋[1-2]、湖泊[3-7]、土壤[8-10]、泥炭[11-13]以及温泉[14]等,其分布在开展古气候变化研究方面显示出了优越性,成为重建过去大陆温度和水文变化的有效手段之一。来源于古菌的类异戊二烯GDGTs(简称isoGDGTs)主要包括GDGT-0~GDGT-3,泉古菌醇(crenarchaeol)及其异构体(crenarchaeol′),其中基于含环戊烷isoGDGTs相对丰度建立的TEX86指标被用于指示过去海水表面温度(SST)[15]。支链GDGTs(简称brGDGTs)来自细菌,其烷基链上具有不同数量、位置的甲基和环戊烷(图 1),被认为由兼性厌氧异养细菌合成[11]。最早在2007年,Weijers等首次根据全球表层土壤brGDGTs分布定义了MBT和CBT指数,并将MBT/CBT指标作为古温度计[8]。在此基础上,Peterse等将全球表层土壤数据集扩展,把土壤中含量丰富的brGDGTs化合物与年平均温度进行拟合,建立了新的MBT′/CBT温度转换公式[9]。但随后De Jonge等发现6-甲基brGDGTs使MBT′/CBT在干旱地区的温度校正存在较大偏差,因此,定义了MBT′5ME指数,提高了重建温度的准确性[16]。截至当前,brGDGTs相关指标已运用于土壤[17]、湖泊[18-20]、泥炭[12-13, 21-22]等沉积物的古气候重建。
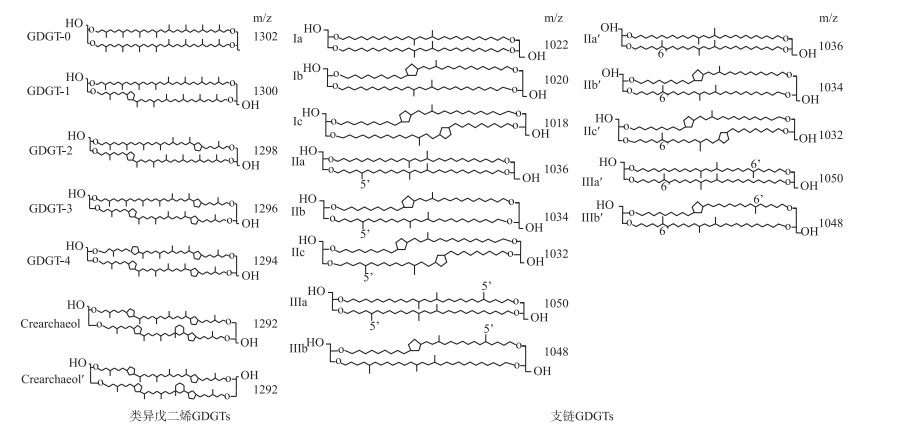
|
图 1 GDGT化合物结构图 Fig.1 Structure of GDGTs compounds |
然而,湖泊沉积物brGDGTs来源复杂,既可以在原位水柱中产生[23-24],又可以接受土壤输入[25-28],将基于土壤的校正公式应用于以原位生产brGDGTs为主的湖泊沉积物时,重建的温度远低于实际值[29],因而在东非湖泊[30-31]、中国湖泊[5, 25, 28]乃至南北极湖泊[7, 32]等都建立了适合湖泊原位生产的温度转换公式。因此,在进行湖泊brGDGTs古气候研究时,明确brGDGTs来源至关重要。
此外,多项研究表明除温度、土壤pH之外[33-35],其他环境因素也控制着土壤和湖泊中GDGTs分布[34, 36]。比如,在部分研究中,CBT指数与含水量或年均降水量相关性较好,可以用于重建湖泊水位变化或作为古降雨替代指数[34, 37]。Pei等发现在土壤中MBT′指数随着氧气水平的降低而增加,CBT和IR6me在低氧环境中比值较低[38],在湖泊中,MBT′与MBT′6ME指数与氧含量表现出强相关性[39]。盐度增加也会影响brGDGTs分布,使得MBT′指数偏高,在温度重建之前需要校正盐分效应[40]。为此,本研究对北方干旱地区察汗淖尔表层样品GDGTs进行系统分析,揭示不同沉积环境GDGTs影响因素,为过去气候变化研究提供基础资料。
1 材料和方法 1.1 样品采集察汗淖尔(41°29′35″N,113°54′12″E)(图 2)位于河北省张家口市尚义县和内蒙古自治区商都县交界处,是亚洲区域季风边缘带,对季风响应敏感,流域面积可达53 km2,是华北地区现存最大的内陆咸水湖。2017年以来,由于气候干旱,地下水过度开采,湿地面积逐渐缩小,至今湖床已全部裸露于地表,只有在夏季雨水充沛时,可短暂形成小型水面。2021年8月,在察汗淖尔流域范围内共取得24个表层样品(2~6 cm),其中湖泊表层沉积物8个,湖泊周围表层土壤16个。利用全球定位系统(GPS)对每个采样地点的经纬度进行记录,根据中国气象数据网(http://data.cma.cn/)公布的气候月数据集得到该地区年平均温度为4℃左右,年降水量在400 mm,主要集中在7、8月(图 3),由于察汗淖尔湖床已暴露于地表且植被覆盖率低,可以近似认为表层沉积物温度与大气温度一致。
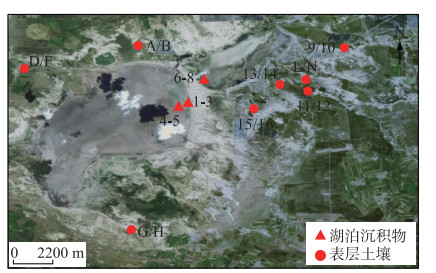
|
图 2 察汗淖尔采样点示意 Fig.2 The sampling sites location of Lake Chahannaoer |

|
图 3 察汗淖尔月均温度(点线图)及降水量(柱状图) (来自1981—2010年中国气象局网站记录的数据集) Fig.3 Monthly mean temperature (Point-Line Chart) and precipitation (Histogram) of Chahannaoer from 1981 to 2010 (Dataset from the website of China Meteorological Administration) |
根据Weijers等的方法测量pH值,将样品与超纯水以1 ∶2.5比例充分混合,经离心机分离后提取上清液,再使用梅特勒托利多pH计进行pH值测定,共测定3次,取平均值,测量误差在±0.03之内。土壤含水量利用差量法进行测定,称量一定质量的样品(m1),将样品放置在冷冻干燥机干燥48 h,再次称量样品质量(m2),则样品含水量(SWC)=(m1-m2)/m2。
1.3 GDGTs抽提与测定将冷冻干燥后的样品研磨成粉末(20 g左右),在室温下,使用二氯甲烷∶甲醇(DCM ∶MeOH)混合溶剂(9 ∶1/V ∶V)在快速溶剂萃取仪中抽取总脂质提取物,并通过旋转蒸发仪浓缩至1~2 mL。分别将正己烷(Hexane)和甲醇(MeOH)作为洗脱液,在硅胶柱内分离提取物的非极性组分和极性组分,收集后的组分在氮气流中进行干燥。使用正己烷溶解极性组分后通过0.45 μm聚四氟乙烯(PTFE)过滤器过滤,在氮吹仪下干燥过滤后的组分,最后定容至500或1000 μL,以待进行后续测试分析,前处理在西北大学地质学系完成。GDGTs测试在中国科学院地球环境研究所完成,使用的是岛津三重串联四极杆液相色谱质谱联用仪,单个样品总运行时间为2 h,进样量为50 μL,分析时加入10 μL的内标物(C46),在单离子扫描模式下对质子化离子进行扫描,通过测定各个化合物离子的峰面积对brGDGTs和isoGDGTs不同组分进行定量分析,根据内标物的丰度计算得到各组分的相对丰度。
1.4 计算公式结合土壤类型和GDGTs丰度分布特征,本文选择了如下计算公式; 所有样品中IIIb和IIIc含量较少,因此使用Peterse等定义的MBT′和CBT指数计算GDGTs的甲基化和环化程度[9]:
| MBT′=(Ia+Ib+Ic)/(Ia+Ib+Ic+IIa+IIa′+IIb+III′+IIc+IIc′+IIIa+IIIa′) | (1) |
| CBT=−lg[(Ib++Ilb2+Ilb′)/(Ia+IIa+IIa′)] | (2) |
| МАAT =0.81−5.67CBT+31.0MBT′ | (3) |
| pH=7.90−1.97CBT | (4) |
De Jonge等将MBT′中与土壤pH值强相关的6-甲基brGDGTs排除,得到的MBT′5ME与温度相关性更好,并且新修订的CBT′指数与pH值在全球土壤中具有最好的相关性,公式如下[16]:
| MBT′5ME=(Ia+Ib+Ic)/(Ia+Ib+Ic+IIa+IIb+IIc+IIIa) | (5) |
| CBT′=lg[(II2+IIa′+IIb′+IIc′+IIIa′+IIIb′+IIIc′)/(Ia+IIa+IIIa)] | (6) |
| MAAT=−8.71+31.45MBT′5ME | (7) |
| pH=7.15+1.59CBT′ | (8) |
此外,我们还应用了Yang等针对中国干旱地区土壤提出的温度校正公式[41],以及Wang等建立的适合北方土壤的区域校正[42]:
| MAAT (Yang )=20.9−13.4f(IIa′IIa′)−17.2f(IIIa′+III′)−17.5f(IIb)+11.2f(Ib) | (9) |
| MAAT(Wang )=32.65MBT′5ME−14.97 | (10) |
| pH(Wang )=1.65CBT′+6.93 | (11) |
根据Sinninghe Damsté等提出的公式计算四甲基化、五甲基化和六甲基化brGDGTs丰度[43],用于分析brGDGTs来源:
| Tetramethylated brGDGTs=Ia+Ib+Ic | (12) |
| Pentamethylated brGDGTs =IIa+IIb+IIc+IIa′+IIb′+IIc′ | (13) |
| Hexamethylated brGDGTs = IIIa + IIIb + IIIc + IIII′+IIII ′+IIII′ | (14) |
使用De Jonge等定义的CI指数以评估生物群落对brGDGTs分布的影响[44]:
| CI=Ia/(Ia+IIa+IIIa) | (15) |
所有样品均检测到丰富的isoGDGTs和brGDGTs,总含量在1.50~29.22 ng/g之间。如图 4所示,湖泊表层沉积物与土壤呈现相似的分布特征,isoGDGTs含量分别在0.14~2.45、0.07~11.14 ng/g之间,主要以crenarchaeol为主(平均占比为48%),其次是GDGT-0(29%)、GDGT-2(7%)、GDGT-1(6%)、crenarchaeol′(5%)和GDGT-3(4%)。
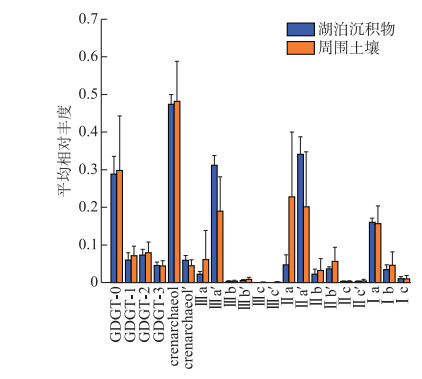
|
图 4 察汗淖尔湖泊及其周围表层土壤GDGTs分布(误差棒为标准偏差) Fig.4 Distribution of GDGTs in surface sediments and surrounding soils of Lake Chahannaoer (Error bars indicate the standard deviation) |
大部分样品brGDGTs丰度较高,平均占GDGTs总量的80% 以上。湖泊沉积物中brGDGTs含量在1.60~13.27 ng/g之间,周围土壤中总含量是0.24~18.08 ng/g。brGDGTs中五甲基化brGDGTs含量最多(图 4),其次是六甲基化和四甲基化brGDGTs。IIa及IIa′占湖泊沉积物brGDGTs的36% ~41%,但在表层土壤中占32% ~51%。
在所有样品中,IIIa′丰度普遍高于其异构体IIIa。而对于五甲基化brGDGTs,在湖泊表层沉积物中,IIa′含量(分别为34%)高于IIa(5%),但土壤中IIa′ (20%)低于IIa (23%)。所有样品中其他化合物相对丰度较低,多数样品无法检测到IIIc、IIIc′。综上所述,表层沉积物和土壤brGDGTs分布基本相似,主要以Ⅱ型为主,然而,IIa和IIa′在沉积物和土壤中的优势地位不同。
2.3 BrGDGTs相关指标湖泊表层沉积物的MBT′变化范围较小,在0.18~0.25之间,CBT指标变化范围较大,介于0.58~1.04(图 5);周围土壤的MBT′值范围是0.10~0.32,CBT数值为0.27~1.16。MBT′5ME在湖泊表层沉积物和周围土壤中的范围分别是0.58~0.83和0.15~0.89;湖泊表层沉积物的CBT′在0.35~0.63之间变化,周围土壤的CBT′指标取值范围在-0.58~0.69之间。
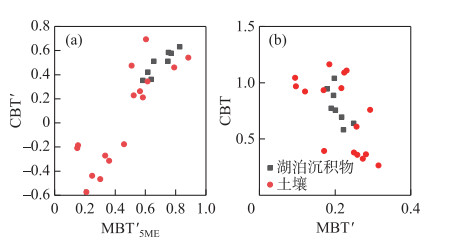
|
图 5 湖泊表层沉积物和土壤中MBT′5ME和CBT′、MBT′和CBT指标交叉图 Fig.5 Cross plots of MBT′5ME and CBT′, MBT′ and CBT for surface sediments and soils |
GDGT-0是isoGDGTs中来源最广泛的化合物,它可由奇古菌(Thaumarchaeota)、泉古菌(Crenarchaeota)和广古菌(Euryarchaeota)等古菌群落合成[45],其中产甲烷菌是GDGT-0的主要贡献源[46],而crenarchaeol及其异构体是氨氧化奇古菌产生的特征标志物[47],因此GDGT-0/crenarchaeol比值可判断isoGDGTs的主要来源[46]。结果显示,所有样品GDGT-0/crenarchaeol比值在0.2~2之间,说明该地区奇古菌占主要地位。目前已培育出两组奇古菌,一种是在海洋水柱中占主导的GroupⅠ.1a[48-50];另一种是在土壤中可产生更多crenarchaeol′的GroupⅠ.1b[51-52],故可利用crenarchaeol/crenarchaeol′比值区分环境中奇古菌类型。当crenarchaeol/crenarchaeol′比值>25时,奇古菌种类主要是GroupⅠ.1a型,如果比值 < 25,则为GroupⅠ.1b型奇古菌[6]。察汗淖尔所有样品crenarchaeol/crenarchaeol′比值<25,表明研究区内古菌主要为GroupⅠ.1b型奇古菌。
湖泊沉积物brGDGTs既可以是原位水柱产生[23-24],也可以通过河流或周围土壤携带输入产生[53-54]。为了评估这种影响,本研究基于察汗淖尔所有样品brGDGTs丰度绘制了三元图(图 6),并与中国北方表层土壤和青海湖等数据进行对比[42, 55]。如图 6所示,察汗淖尔样品具有较丰富的五甲基化brGDGTs,以及相对较少的四甲基和六甲基brGDGTs,显示了与北方表层土壤相似的分布模式,而具有原位brGDGTs来源的湖泊沉积物中六甲基brGDGTs相对丰富。此外,brGDGTs相关指标的交叉图(图 5)显示, 周围土壤的MBT′、CBT、MBT′5ME以及CBT′指标数值变化范围较大,而湖泊表层沉积物中相关指标数值变化范围相对较小,两者没有明显差异。上述结果表明察汗淖尔湖泊表层沉积物中brGDGTs可能主要来源于周围土壤,由于缺乏水柱样品,无法确定原位生产对brGDGTs分布的影响。
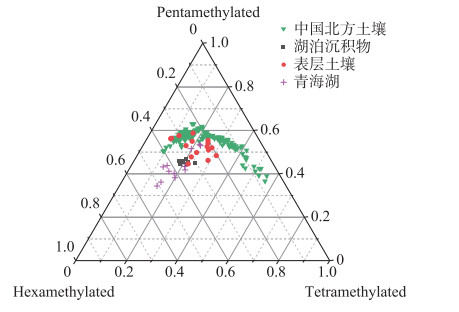
|
图 6 察汗淖尔样品四甲基化、五甲基化和六甲基化brGDGTs丰度三元图,并与中国北方土壤和青海湖等表层沉积物数据比较[42, 55] Fig.6 Ternary diagram of the fractional abundances of tetramethylated, pentamethylated, and hexamethylated brGDGTs from the different sample sets[42, 55] |
多项研究表明pH值、含水量是影响湖泊沉积物brGDGTs分布的重要因素[8, 34, 36-37, 47, 56-60],可利用RDA分析检验这些环境参数对察汗淖尔brGDGTs分布的贡献。结果如图 7所示,前两个RDA轴记录了brGDGTs分布受土壤含水量影响较小(P=0.06),主要由土壤pH值控制(P=0.02)。土壤pH值与IIIa′、IIa、Ia丰度呈正相关,与IIIa、IIa′呈负相关,造成了CBT与土壤pH值之间的相关性较弱(R=0.1,图 7)。这一现象也与其他研究相符,如青藏高原的表层土壤碱性越强,pH值与CBT的相关关系越趋于平缓[61];对于中国北方的碱性土壤(pH>8),CBT与pH值的正相关性不显著[42]。本文所有样品pH在7.9~9.2之间变化,碱性较强,这可能是导致pH值与CBT不具有相关性的原因之一。
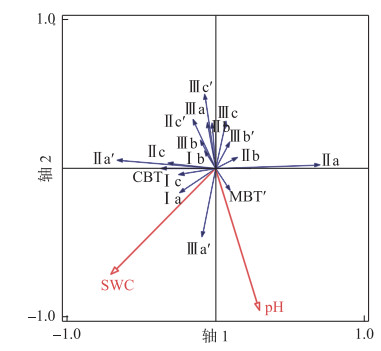
|
图 7 BrGDGTs冗余分析(RDA)图,显示了现有环境因子(红色箭头)与brGDGTs化合物及相关指数(蓝色箭头)之间的关系 Fig.7 Redundancy analysis (RDA) showing relationships between existing environment variables (red arrows) and compounds and indexes of brGDGTs (blue arrows) |
察汗淖尔湖泊表层沉积物及其周围土壤brGDGTs与北方表层土壤分布较为一致,因此在重建温度和pH值时使用基于土壤的校准公式更合适(图 8)。考虑到IIa和IIa′在湖泊沉积物和土壤中优势地位不同,需将两者的温度和pH值分别进行计算。在湖泊沉积物中,当使用原始全球土壤校准公式(公式(3))时,重建的大气温度范围在1.0~4.9℃,平均为2.7℃,接近年均温度4.0℃;重建的土壤pH值(公式(4))为5.8~6.8,平均是6.3,低于平均测量pH值(8.9)。使用修订后的全球土壤校准公式(公式(7))的计算结果介于9.8~17.5℃,平均温度为13.4℃,同样高于预期值;计算的pH值(公式(8))范围在7.7~8.2之间,平均为7.9,与实际测量值相比偏低。当应用干旱地区土壤校准时(公式(9)),重建温度为7.5~11.9℃,平均是9.8℃,显然高于观测温度。运用北方区域温度校准公式(公式(10))得到的结果为4.1~12.1℃,平均温度是7.8℃,略低于器测温度(4℃);重建的土壤pH值(公式(11))在7.5~8.0之间,均低于测量值。
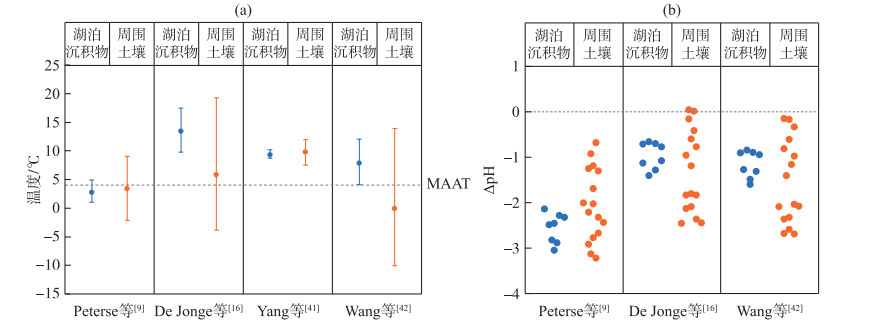
|
图 8 基于不同土壤校准公式计算的大气温度(a)和土壤pH误差值(b) (ΔpH是CBT-pH计算值与测量值的差值) Fig.8 Comparison of reconstructed temperature based on different soil calibrations (a) and the differences(ΔpH) between CBT-inferred pH and the measured pH (b) |
而对于土壤,使用原始MBT′/CBT校准(公式(3))的计算结果为-2.1~9.1℃,平均为3.4℃,略低于器测记录温度(4℃);重建的土壤pH值(公式(4))为5.7~7.4,均低于平均测量值(pH=8.5)。应用修订后的土壤校准公式(公式(7))重建的温度为-3.8~19.2℃,平均是5.8℃;计算的土壤pH值(公式(8))范围在6.2~8.2之间,普遍低于实际测量值。利用Yang等建立的干旱地区校准公式(公式(9))得到的结果是7.5~12.0℃,平均温度为9.8℃,远高于年均温度4℃[41]。北方区域校准公式(公式(10))重建的温度范围较大,介于-10.0~14.0℃之间,平均为-0.1℃;重建的土壤pH值(公式(11))为6.0~8.1,与测量值相比偏低。
综上所述,使用全球MBT′/CBT土壤校准[9]得到的结果较为接近记录的年均温度,但仍存在一定偏差,区域校准结果并不比全球土壤校准更精确。值得注意的是,使用针对干旱环境或消除了5-Me和6-Me异构体影响的最新校准获得的温度结果偏高,这与其他干旱、寒冷地区情况一致[58, 62],可能是由于在这种特殊的寒冷地区,brGDGTs生产细菌倾向于在夏季活跃[28, 63]。
为了进一步探究重建温度产生偏移的原因,本研究利用CI指数评估细菌群落对brGDGTs分布的影响[44]。De Jonge等认为土壤brGDGTs分布随微生物群落变化而变化,并依据冷簇和暖簇细菌群落脂质将全球土壤数据划分为暖集群(CI>0.64)和冷集群(CI<0.64)[44]。经计算,我们发现察汗淖尔湖泊表层沉积物和土壤细菌群落基本与全球干旱土壤一致,均属于冷簇(图 9)。以往研究表明,多数冷簇土壤细菌brGDGTs分布与温度相关性较弱或不显著[44],而主要受土壤pH值控制[42, 58],这与察汗淖尔结果一致。显然,细菌群落组成和土壤pH值可能引起上述重建结果出现偏差。

|
图 9 察汗淖尔和全球干旱地区土壤的群落指数(CI)箱线图 Fig.9 Boxplot of the community index (CI) values for the sediments and soils from Lake Chahannaoer and global arid regions |
本研究对察汗淖尔现代表层样品进行了GDGTs分析,结果显示湖泊沉积物及其周围土壤与北方表层土壤GDGTs分布相似,isoGDGTs以crenarchaeol和GDGT-0为主,brGDGTs中五甲基化brGDGTs(IIa、IIa′)丰度最高,但IIa和IIa′在沉积物和土壤中优势地位不同。GDGT-0/crenarchaeol比值介于0~2之间,crenarchaeol与其异构体比值小于25,说明isoGDGTs主要由GroupⅠ.1b型奇古菌产生。土壤pH值是影响brGDGTs分布的主要环境因素,CI指数显示研究区产brGDGTs细菌属于冷簇,与温度相关性较弱或不显著,这些造成了应用多种温度校准公式的计算结果与器测记录不一致,重建的土壤pH值偏低。因此,在这种特殊的寒冷干旱环境中,GDGTs分布较为复杂,需谨慎选择校正公式以获得准确的气候信息。
致谢: 感谢中国科学院地球环境研究所对实验工作提供的支持。
| [1] |
Karner MB, DeLong EF, Karl DM. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature, 2001, 409(6819): 507-510. DOI:10.1038/35054051 |
| [2] |
Yao P, Yuan ZG. Advances of intact polar membrane lipids as chemical biomarkers for extant microorganisms in marine sediments. Advances in Earth Science, 2010, 25: 474-483. DOI:10.11867/J.ISSN.1001-8166.2010.05.0474 |
| [3] |
Powers L, Werne JP, Vanderwoude AJ et al. Applicability and calibration of the TEX86 paleothermometer in lakes. Organic Geochemistry, 2010, 41(4): 404-413. DOI:10.1016/j.orggeochem.2009.11.009 |
| [4] |
Woltering M, Johnson TC, Werne JP et al. Late Pleistocene temperature history of Southeast Africa: A TEX86 temperature record from Lake Malawi. Palaeogeography, Palaeoclimatology, Palaeoecology, 2011, 303(1/2/3/4): 93-102. DOI:10.1016/j.palaeo.2010.02.013 |
| [5] |
Qian S, Yang H, Dong CH et al. Rapid response of fossil tetraether lipids in lake sediments to seasonal environmental variables in a shallow lake in central China: Implications for the use of tetraether-based proxies. Organic Geochemistry, 2019, 128: 108-121. DOI:10.1016/j.orggeochem.2018.12.007 |
| [6] |
Li JJ, Pancost RD, Naafs BDA et al. Distribution of glycerol dialkyl glycerol tetraether (GDGT) lipids in a hypersaline lake system. Organic Geochemistry, 2016, 99: 113-124. DOI:10.1016/j.orggeochem.2016.06.007 |
| [7] |
Foster LC, Pearson EJ, Juggins S et al. Development of a regional glycerol dialkyl glycerol tetraether (GDGT)-temperature calibration for Antarctic and sub-Antarctic lakes. Earth and Planetary Science Letters, 2016, 433: 370-379. DOI:10.1016/j.epsl.2015.11.018 |
| [8] |
Weijers JWH, Schouten S, van den Donker JC et al. Environmental controls on bacterial tetraether membrane lipid distribution in soils. Geochimica et Cosmochimica Acta, 2007, 71(3): 703-713. DOI:10.1016/j.gca.2006.10.003 |
| [9] |
Peterse F, van der Meer J, Schouten S et al. Revised calibration of the MBT-CBT paleotemperature proxy based on branched tetraether membrane lipids in surface soils. Geochimica et Cosmochimica Acta, 2012, 96: 215-229. DOI:10.1016/j.gca.2012.08.011 |
| [10] |
Xiao W, Xu Y, Ding S et al. Global calibration of a novel, branched GDGT-based soil pH proxy. Organic Geochemistry, 2015, 89/90: 56-60. DOI:10.1016/j.orggeochem.2015.10.005 |
| [11] |
Weijers JWH, Schouten S, Hopmans EC et al. Membrane lipids of mesophilic anaerobic bacteria thriving in peats have typical archaeal traits. Environ Microbiol, 2006, 8(4): 648-657. DOI:10.1111/j.1462-2920.2005.00941.x |
| [12] |
Zheng YH, Li QY, Wang ZZ et al. Peatland GDGT records of Holocene climatic and biogeochemical responses to the Asian Monsoon. Organic Geochemistry, 2015, 87: 86-95. DOI:10.1016/j.orggeochem.2015.07.012 |
| [13] |
Zheng YH, Pancost RD, Liu XD et al. Atmospheric connections with the North Atlantic enhanced the deglacial warming in northeast China. Geology, 2017, 45(11): 1031-1034. DOI:10.1130/g39401.1 |
| [14] |
Xie W, Zhang CL, Wang JX et al. Distribution of ether lipids and composition of the archaeal community in terrestrial geothermal springs: Impact of environmental variables. Environmental Microbiology, 2015, 17(5): 1600-1614. DOI:10.1111/1462-2920.12595 |
| [15] |
Schouten S, Hopmans EC, Schefuß E et al. Distributional variations in marine crenarchaeotal membrane lipids: A new tool for reconstructing ancient sea water temperatures?. Earth and Planetary Science Letters, 2002, 204(1/2): 265-274. DOI:10.1016/S0012-821X(02)00979-2 |
| [16] |
De Jonge C, Hopmans EC, Zell CI et al. Occurrence and abundance of 6-methyl branched glycerol dialkyl glycerol tetraethers in soils: Implications for palaeoclimate reconstruction. Geochimica et Cosmochimica Acta, 2014, 141: 97-112. DOI:10.1016/j.gca.2014.06.013 |
| [17] |
Sinninghe Damsté JS, Ossebaar J, Schouten S et al. Altitudinal shifts in the branched tetraether lipid distribution in soil from Mt. Kilimanjaro (Tanzania): Implications for the MBT/CBT continental palaeothermometer. Organic Geochemistry, 2008, 39(8): 1072-1076. DOI:10.1016/j.orggeochem.2007.11.011 |
| [18] |
Ajioka T, Yamamoto M, Murase J. Branched and isoprenoid glycerol dialkyl glycerol tetraethers in soils and lake/river sediments in Lake Biwa Basin and implications for MBT/CBT proxies. Organic Geochemistry, 2014, 73: 70-82. DOI:10.1016/j.orggeochem.2014.05.009 |
| [19] |
Wang MD, Liang J, Hou JZ et al. Distribution of GDGTs in lake surface sediments on the Tibetan Plateau and its influencing factors. Science China Earth Sciences, 2016, 59(5): 961-974. DOI:10.1007/s11430-015-5214-3 |
| [20] |
Hu JF, Zhou HD, Peng PA et al. Seasonal variability in concentrations and fluxes of glycerol dialkyl glycerol tetraethers in Huguangyan Maar Lake, SE China: Implications for the applicability of the MBT-CBT paleotemperature proxy in lacustrine settings. Chemical Geology, 2016, 420: 200-212. DOI:10.1016/j.chemgeo.2015.11.008 |
| [21] |
Li Q, Liu X, Wang Z et al. Distrubutions and environmental significance of GDGTs in modren past samples from eastern Tibetan Plateau. Quaternary Sciences, 2016, 36(2): 388-395. |
| [22] |
Naafs BDA, Inglis GN, Zheng Y et al. Introducing global peat-specific temperature and pH calibrations based on brGDGT bacterial lipids. Geochimica et Cosmochimica Acta, 2017, 208: 285-301. DOI:10.1016/j.gca.2017.01.038 |
| [23] |
Tierney JE, Russell JM. Distributions of branched GDGTs in a tropical lake system: Implications for lacustrine application of the MBT/CBT paleoproxy. Organic Geochemistry, 2009, 40(9): 1032-1036. DOI:10.1016/j.orggeochem.2009.04.014 |
| [24] |
Bechtel A, Smittenberg RH, Bernasconi SM et al. Distribution of branched and isoprenoid tetraether lipids in an oligotrophic and a eutrophic Swiss Lake: Insights into sources and GDGT-based proxies. Organic Geochemistry, 2010, 41(8): 822-832. DOI:10.1016/j.orggeochem.2010.04.022 |
| [25] |
Sun Q, Chu G, Liu M et al. Distributions and temperature dependence of branched glycerol dialkyl glycerol tetraethers in recent lacustrine sediments from China and Nepal. Journal of Geophysical Research, 2011, 116(G1). DOI:10.1029/2010jg001365 |
| [26] |
Loomis SE, Russell JM, Heureux AM et al. Seasonal variability of branched glycerol dialkyl glycerol tetraethers (brGDGTs) in a temperate lake system. Geochimica et Cosmochimica Acta, 2014, 144: 173-187. DOI:10.1016/j.gca.2014.08.027 |
| [27] |
Naeher S, Peterse F, Smittenberg RH et al. Sources of glycerol dialkyl glycerol tetraethers (GDGTs) in catchment soils, water column and sediments of Lake Rotsee (Switzerland)—Implications for the application of GDGT-based proxies for lakes. Organic Geochemistry, 2014, 66: 164-173. DOI:10.1016/j.orggeochem.2013.10.017 |
| [28] |
Dang X, Ding W, Yang H et al. Different temperature dependence of the bacterial brGDGT isomers in 35 Chinese lake sediments compared to that in soils. Organic Geochemistry, 2018, 119: 72-79. DOI:10.1016/j.orggeochem.2018.02.008 |
| [29] |
Blaga CI, Reichart GJ, Schouten S et al. Branched glycerol dialkyl glycerol tetraethers in lake sediments: Can they be used as temperature and pH proxies?. Organic Geochemistry, 2010, 41(11): 1225-1234. DOI:10.1016/j.orggeochem.2010.07.002 |
| [30] |
Loomis SE, Russell JM, Ladd B et al. Calibration and application of the branched GDGT temperature proxy on East African Lake sediments. Earth and Planetary Science Letters, 2012, 357/358: 277-288. DOI:10.1016/j.epsl.2012.09.031 |
| [31] |
Russell JM, Hopmans EC, Loomis SE et al. Distributions of 5- and 6-methyl branched glycerol dialkyl glycerol tetraethers (brGDGTs) in East African Lake sediment: Effects of temperature, pH, and new lacustrine paleotemperature calibrations. Organic Geochemistry, 2018, 117: 56-69. DOI:10.1016/j.orggeochem.2017.12.003 |
| [32] |
Peterse F, Vonk JE, Holmes RM et al. Branched glycerol dialkyl glycerol tetraethers in Arctic Lake sediments: Sources and implications for paleothermometry at high latitudes. Journal of Geophysical Research: Biogeosciences, 2014, 119(8): 1738-1754. DOI:10.1002/2014jg002639 |
| [33] |
Zech R, Gao L, Tarozo R et al. Branched glycerol dialkyl glycerol tetraethers in Pleistocene loess-paleosol sequences: Three case studies. Organic Geochemistry, 2012, 53: 38-44. DOI:10.1016/j.orggeochem.2012.09.005 |
| [34] |
Wang H, Liu W, Zhang CL. Dependence of the cyclization of branched tetraethers on soil moisture in alkaline soils from arid-subhumid China: Implications for palaeorainfall reconstructions on the Chinese Loess Plateau. Biogeosciences, 2014, 11(23): 6755-6768. DOI:10.5194/bg-11-6755-2014 |
| [35] |
Menges J, Huguet C, Alcañiz JM et al. Influence of water availability in the distributions of branched glycerol dialkyl glycerol tetraether in soils of the Iberian Peninsula. Biogeosciences, 2014, 11(10): 2571-2581. DOI:10.5194/bg-11-2571-2014 |
| [36] |
Dang XY, Yang H, Naafs BDA et al. Evidence of moisture control on the methylation of branched glycerol dialkyl glycerol tetraethers in semi-arid and arid soils. Geochimica et Cosmochimica Acta, 2016, 189: 24-36. DOI:10.1016/j.gca.2016.06.004 |
| [37] |
Cao JT, Rao ZG, Shi FX et al. Lake-level records support a mid-Holocene maximum precipitation in Northern China. Science China Earth Sciences, 2021, 64(12): 2161-2171. DOI:10.1007/s11430-020-9833-3 |
| [38] |
Pei HY, Zhao SJ, Yang H et al. Variation of branched tetraethers with soil depth in relation to non-temperature factors: Implications for paleoclimate reconstruction. Chemical Geology, 2021, 572: 120211. DOI:10.1016/j.chemgeo.2021.120211 |
| [39] |
Wu J, Yang H, Pancost RD et al. Variations in dissolved O2 in a Chinese Lake drive changes in microbial communities and impact sedimentary GDGT distributions. Chemical Geology, 2021, 579: 120348. DOI:10.1016/j.chemgeo.2021.120348 |
| [40] |
Wang HY, Liu WG, He YX et al. Salinity-controlled isomerization of lacustrine brGDGTs impacts the associated MBT'5ME terrestrial temperature index. Geochimica et Cosmochimica Acta, 2021, 305: 33-48. DOI:10.1016/j.gca.2021.05.004 |
| [41] |
Yang H, Pancost RD, Dang XY et al. Correlations between microbial tetraether lipids and environmental variables in Chinese soils: Optimizing the paleo-reconstructions in semi-arid and arid regions. Geochimica et Cosmochimica Acta, 2014, 126: 49-69. DOI:10.1016/j.gca.2013.10.041 |
| [42] |
Wang HY, Liu WG, Lu HX. Appraisal of branched glycerol dialkyl glycerol tetraether-based indices for North China. Organic Geochemistry, 2016, 98: 118-130. DOI:10.1016/j.orggeochem.2016.05.013 |
| [43] |
Sinninghe Damsté JS. Spatial heterogeneity of sources of branched tetraethers in shelf systems: The geochemistry of tetraethers in the Berau River delta (Kalimantan, Indonesia). Geochimica et Cosmochimica Acta, 2016, 186: 13-31. DOI:10.1016/j.gca.2016.04.033 |
| [44] |
De Jonge C, Radujković D, Sigurdsson BD et al. Lipid biomarker temperature proxy responds to abrupt shift in the bacterial community composition in geothermally heated soils. Organic Geochemistry, 2019, 137. DOI:10.1016/j.orggeochem.2019.07.006 |
| [45] |
Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proceedings of the National Academy of Sciences of the United States of America, 1990, 87(12): 4576-4579. DOI:10.1073/pnas.87.12.4576 |
| [46] |
Blaga CI, Reichart GJ, Heiri O et al. Tetraether membrane lipid distributions in water-column particulate matter and sediments: A study of 47 European Lakes along a north-south transect. Journal of Paleolimnology, 2009, 41(3): 523-540. DOI:10.1007/s10933-008-9242-2 |
| [47] |
Schouten S, Hopmans EC, Sinninghe Damsté JS. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review. Organic Geochemistry, 2013, 54: 19-61. DOI:10.1016/j.orggeochem.2012.09.006 |
| [48] |
Schouten S, Hopmans EC, Baas M et al. Intact membrane lipids of Candidatus Nitrosopumilus maritimus, a cultivated representative of the cosmopolitan mesophilic group I Crenarchaeota. Applied and Environmental Microbiology, 2008, 74(8): 2433-2440. DOI:10.1128/AEM.01709-07 |
| [49] |
Pitcher A, Hopmans EC, Mosier AC et al. Core and intact polar glycerol dibiphytanyl glycerol tetraether lipids of ammonia-oxidizing Archaea enriched from marine and estuarine sediments. Applied and Environmental Microbiology, 2011, 77(10): 3468-3477. DOI:10.1128/AEM.02758-10 |
| [50] |
Jung MY, Park SJ, Min D et al. Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural soil. Applied and Environmental Microbiology, 2011, 77(24): 8635-8647. DOI:10.1128/AEM.05787-11 |
| [51] |
Pitcher A, Rychlik N, Hopmans EC et al. Crenarchaeol dominates the membrane lipids of Candidatus Nitrososphaera gargensis, a thermophilic Group I.1b Archaeon. The ISME Journal, 2010, 4(4): 542-552. DOI:10.1038/ismej.2009.138 |
| [52] |
Damsté JS, Rijpstra WI, Hopmans EC et al. Intact polar and core glycerol dibiphytanyl glycerol tetraether lipids of group I.1a and I.1b thaumarchaeota in soil. Applied and Environmental Microbiology, 2012, 78(19): 6866-6874. DOI:10.1128/AEM.01681-12 |
| [53] |
Wang HY, Liu WG, Zhang CL et al. Distribution of glycerol dialkyl glycerol tetraethers in surface sediments of Lake Qinghai and surrounding soil. Organic Geochemistry, 2012, 47: 78-87. DOI:10.1016/j.orggeochem.2012.03.008 |
| [54] |
Günther F, Thiele A, Gleixner G et al. Distribution of bacterial and archaeal ether lipids in soils and surface sediments of Tibetan lakes: Implications for GDGT-based proxies in saline high mountain lakes. Organic Geochemistry, 2014, 67: 19-30. DOI:10.1016/j.orggeochem.2013.11.014 |
| [55] |
Zhao C, Rohling EJ, Liu ZY et al. Possible obliquity-forced warmth in southern Asia during the last glacial stage. Science Bulletin, 2021, 66(11): 1136-1145. DOI:10.1016/j.scib.2020.11.016 |
| [56] |
Peterse F, Nicol GW, Schouten S et al. Influence of soil pH on the abundance and distribution of core and intact polar lipid-derived branched GDGTs in soil. Organic Geochemistry, 2010, 41(10): 1171-1175. DOI:10.1016/j.orggeochem.2010.07.004 |
| [57] |
Tierney JE, Russell JM, Eggermont H et al. Environmental controls on branched tetraether lipid distributions in tropical East African Lake sediments. Geochimica et Cosmochimica Acta, 2010, 74(17): 4902-4918. DOI:10.1016/j.gca.2010.06.002 |
| [58] |
Duan YW, Sun Q, Werne JP et al. Soil pH dominates the distributions of both 5- and 6-methyl branched tetraethers in arid regions. Journal of Geophysical Research: Biogeosciences, 2020, 125(10). DOI:10.1029/2019jg005356 |
| [59] |
Sun MM, Yang SL, Xiao JL et al. BrGDGTs-based temperature and hydrological reconstruction from fluvio-lacustrine sediments in the monsoonal North China Plain since 31 kyr BP. Quaternary Science Reviews, 2022, 277: 107268. DOI:10.1016/j.quascirev.2021.107268 |
| [60] |
Loomis SE, Russell JM, Sinninghe Damsté JS. Distributions of branched GDGTs in soils and lake sediments from western Uganda: Implications for a lacustrine paleothermometer. Organic Geochemistry, 2011, 42(7): 739-751. DOI:10.1016/j.orggeochem.2011.06.004 |
| [61] |
Xie S, Pancost RD, Chen L et al. Microbial lipid records of highly alkaline deposits and enhanced aridity associated with significant uplift of the Tibetan Plateau in the Late Miocene. Geology, 2012, 40(4): 291-294. DOI:10.1130/g32570.1 |
| [62] |
Ding S, Xu Y, Wang Y et al. Distribution of branched glycerol dialkyl glycerol tetraethers in surface soils of the Qinghai-Tibetan Plateau: Implications of brGDGTs-based proxies in cold and dry regions. Biogeosciences, 2015, 12(11): 3141-3151. DOI:10.5194/bg-12-3141-2015 |
| [63] |
Raberg JH, Harning DJ, Crump SE et al. Revised fractional abundances and warm-season temperatures substantially improve brGDGT calibrations in lake sediments. Biogeosciences, 2021, 18(12): 3579-3603. DOI:10.5194/bg-18-3579-2021 |
 2024, Vol. 36
2024, Vol. 36 


