摘要
浮游藻类广泛分布于海洋和内陆水体生态系统中,其生长和发育过程具有明显的时空异质性,对气候变化的响应也极为复杂。藻类物候描述了其在长期适应气候、水质和人类干预等因素下的周期性变化,从而形成一种与环境条件相适应的生长发育节律。它主要包括藻类的出现时间、增长高峰以及减少或消亡的时间等特征。遥感技术通过高时空分辨率持续获取叶绿素a浓度数据(浮游藻类生物量的表征参数),实现对藻类物候的长期监测。本文详细地回顾了近年来遥感藻类物候监测和提取方法的进展,指出目前存在的问题与不足,并对未来的发展趋势进行展望。首先,回顾了现有卫星遥感提供大范围时空连续的藻类生长信息。其次,总结了浮游藻类物候阶段的监测,以及估计藻类特定物候阶段的方法。同时,介绍了用于遥感时间序列估算藻类物候的常用数据处理方法,探讨了浮游藻类物候特性的变化趋势。最后,探索了可能影响藻类物候变化的因素和驱动机制。基于以上分析,本文指出未来藻类物候遥感的研究应重点关注:(1)开发并验证适用于不同水域环境的通用算法,结合机器学习等智能算法改进物候模型,提高物候监测精度和算法的业务化应用水平;(2)结合数值模型和生态系统动态模型,深入研究浮游藻类物候背后的驱动机制。
Abstract
Phytoplankton are widely distributed in marine and freshwater ecosystems. Their growth and development show considerable spatial and temporal variation, and their response to climate change is complex. Algal phenology describes the cyclical changes in phytoplankton under long-term adaptation to factors such as climate, water quality and human disturbance, establishing a growth rhythm tuned to environmental conditions. It primarily includes characteristics such as the timing of algal appearance, peak growth and decline or disappearance. Remote sensing technology continuously provides high spatio-temporal resolution data on chlorophyll-a concentrations (an indicator of phytoplankton biomass), allowing long-term monitoring of algal phenology. This paper provided a detailed review of recent advances in remote sensing methods for monitoring and extracting algal phenology, identified current issues and limitations, and looked ahead to future trends. First, it reviewed how existing satellite remote sensing provided comprehensive spatio-temporally continuous information on algal growth. Secondly, it summarized the monitoring of phytoplankton phenological stages and methods for estimating specific algal phenological phases. It also presented common data processing methods used to estimate algal phenology from remote-sensed time series and discussed changing trends in phytoplankton phenological characteristics. Finally, it examined the factors and mechanisms that may influence changes in algal phenology. Based on this analysis, future research on remote sensing of algal phenology should focus on: (1) developing and validating general algorithms suitable for different aquatic environments, integrating machine learning and other intelligent algorithms to improve phenological models and increase the accuracy of phenological monitoring and operational application, (2) combining numerical models with ecosystem dynamics models to investigate the driving mechanisms behind phytoplankton phenology.
Keywords
浮游藻类在水体初级生产力中占主导地位,能反映生态系统变化和气候变化之间的相互影响关系[1]。浮游藻类生长周期快,其生命周期一般在一年内结束,这使得藻类生长的季节变化主要受当年环境的影响[2-3]。但是在过去几十年里,受到全球气候变化以及人类活动的影响,藻类季节性生长的时间和规模发生改变,从而影响水体生态系统的平衡[4-5]。物候学是指对重复的植物和动物生命周期阶段的研究,藻类物候描述了藻类在适应气候、水质及人为活动等影响因素下,其生长和发展所呈现的周期性变化,主要包括藻类的出现时间、增长高峰以及减少或消亡的时间等特征。藻类物候能够揭示藻类生长趋势以及水生态系统的动态变化[6-7]。
藻类物候与水华之间存在密切的关系。水华是藻类在短时间内快速大量繁殖的现象,通常发生在适宜的物候条件下。海洋学家Sverdrup在1953年提出的“临界深度理论”奠定了藻类水华形成的理论基础[8]。当水柱中混合层深度小于临界深度时,藻类总光合作用生产量大于呼吸作用的消耗量,其生物量才能不断累积[9]。然而,近年来气候的变化对藻类物候的影响越发显著。无论是海洋还是内陆水体生态系统,全球范围内藻类物候表现出生长季开始时间提前、结束时间推迟以及生长季延长的趋势[10]。同时,不断变化的水环境因子(如温度、营养盐等)又增加了藻类季节性生长的不确定性[11]。温度和盐度通过影响表层水体的垂直混合结构进而改变混合层的深度,使水体的垂直混合作用发生变化[12]。温度升高能直接增强藻类光合作用和呼吸作用,提高藻类生长及繁殖速率,缩短藻类生物量达到峰值的时间,使物候提前[13-14]。水体中营养盐浓度对水华的发生和发生时间具有显著影响,营养盐(特别是氮和磷)的浓度是驱动水华发生的关键因素。营养盐的浓度和比例(如氮与磷的比例)达到一定阈值后可以直接影响水类的生长速度和生长量,从而间接影响水华的发生时间[15]。随着研究的不断深入,建立了相对完整的藻类物候过程的动力学理论。这些理论综合了藻类生长、繁殖及其与环境因子的相互作用,通过数学模型以及数据分析揭示了不同条件下藻类变化的规律。
叶绿素a(Chl.a)是存在于所有光合藻类中的一种色素,通常被用于表征藻类生物量[16]。Chl.a浓度的变化趋势可以反映水体中藻类的生长和发育过程。Chl.a浓度是藻类物候研究最直接的表征参数[17-18]。目前的研究主要通过海洋水色卫星中的各类传感器来估算Chl.a浓度,以监测藻类物候变化的各项指标[6,19]。Chl.a浓度的变化与海洋和内陆水体不同的气候和物理驱动因素相关联[16]。因此,需要长期通过卫星反演或原位测量的Chl.a数据集来评估浮游藻类物候的季节性和年际变化规律[20]。虽然卫星反演的数据在空间和时间上可能存在局限性,但它能够在全球视角下为我们提供过去几十年藻类物候的变化趋势[19]。
研究藻类物候不仅有助于理解水体生态系统的动态变化,还能为水华的预测和管理提供科学依据。目前对于藻类在复杂环境中物候过程的认知仍然不够全面,尚缺乏系统性的归纳与综述。此外,如何提高藻类物候监测的时空分辨率和准确性一直是藻类物候研究所面临的问题和挑战。为进一步将遥感技术应用于藻类物候研究并为水生态系统管理提供科学指导,本文将系统地回顾藻类物候遥感技术的发展历程、研究方法和驱动因素,并进行全面梳理和总结。
1 藻类物候遥感相关文献来源分析
为全面掌握国内外藻类物候遥感相关研究进展,检索了2009—2024年藻类遥感相关研究论文。英文文献来源于Web of Science的Science Citation Index Expand(SCI-EXPANDED)和Social Sciencs Citation Index(SSCI)核心数据库,以“Phytoplankton”“Remote Sensing”“Phenology”为主题词进行检索;中文文献来源于中国知网(CNKI),以“浮游植物”“遥感”“物候”为关键词检索。文献计量分析显示,“Phenology”“Remote Sensing”“Phytoplankton”是近15年间藻类物候遥感领域发表的SCI索引论文中的3个最常用的关键词(图1a)。在检索到的103篇有效论文中,英文论文98篇,中文论文5篇。自2009年以来,藻类物候遥感发文与被引论文数量总体上呈现上升趋势,特别是近3年,藻类物候研究发展迅速,年发文数量和引文数量增加明显,在2021年和2022年都达到了15篇(图1b),藻类物候成为近年来遥感领域的新兴主题和热点之一[21-22]。
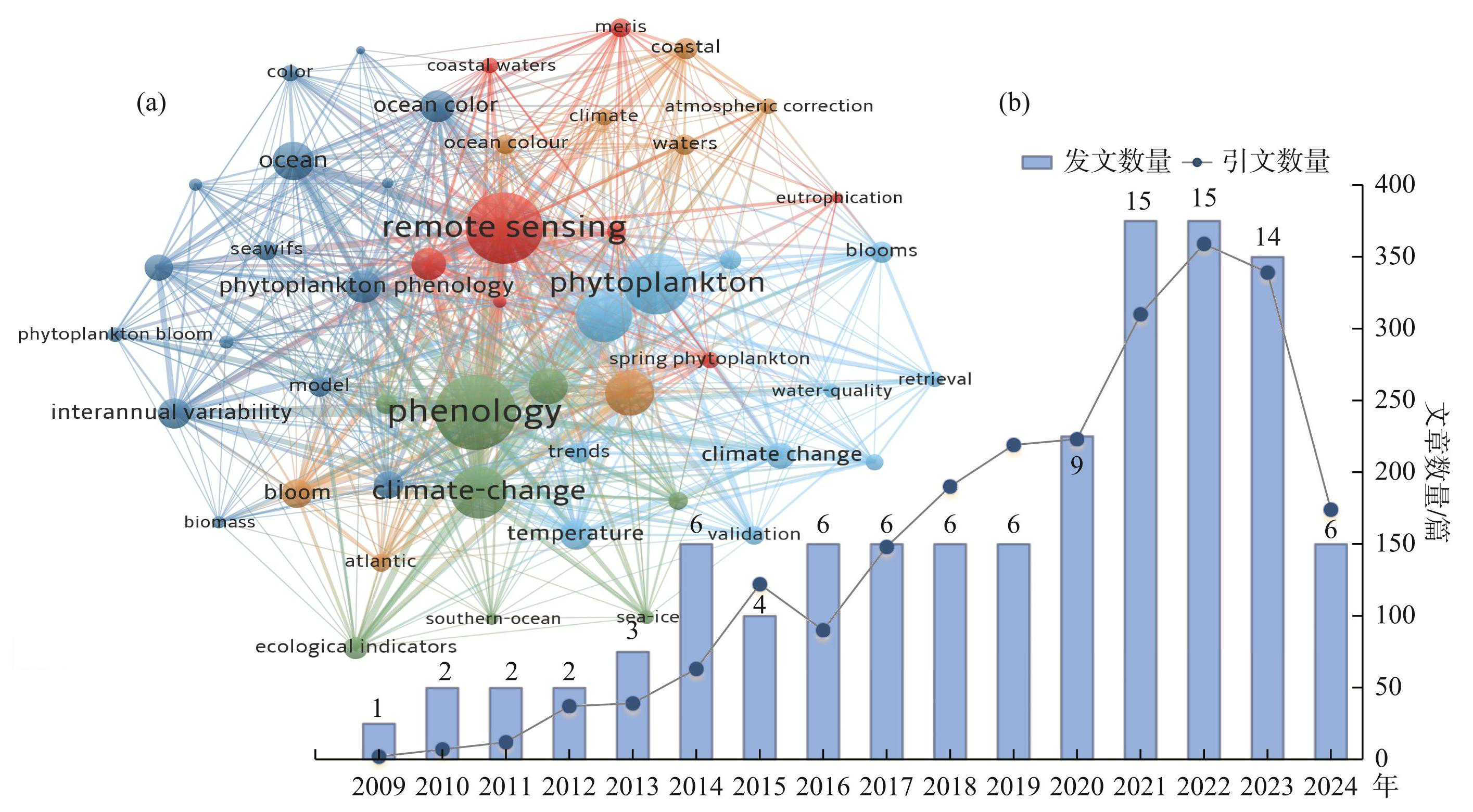
图1(a)以“Phytoplankton”“Remote Sensing”“Phenology”为主题词在Web of science中检索得到的可视化图(关键词越大表示出现频率越高);(b)浮游藻类物候研究发文和引文数量的变化趋势
Fig.1(a) A visualization map retrieved from Web of Science using “Phytoplankton”, “Remote Sensing” and “Phenology” as keywords(the larger the keyword, the higher the frequency of occurrence);(b) Trends in publications and citations on phytoplankton phenology research
2 浮游藻类物候遥感技术发展
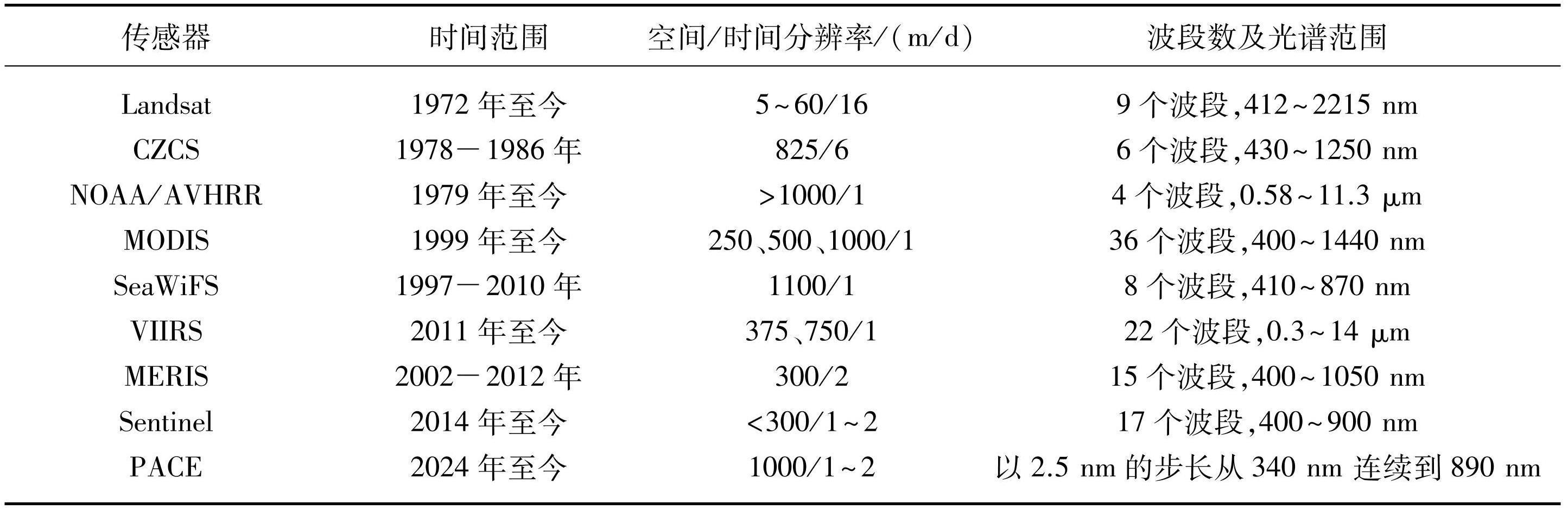
遥感技术具有观察范围广、时间连续性强等特点,能有效识别藻类生长过程的周期性变化。遥感技术的进步使长期监测藻类生长成为可能,极大地推动了对藻类物候学的认识[23]。众多传感器被应用于表层海洋和内陆水体藻类物候的监测之中(表1),例如美国陆地(Landsat)探测卫星系列、宽视场水色扫描仪(SeaWiFS)、沿岸带水色扫描仪(CZCS)、中分辨率成像光谱仪(MODIS)和中分辨率成像光谱仪(MERIS)[6,24-25]等。
在20世纪后期,遥感技术逐渐发展成熟,卫星遥感提供的时间序列数据已经积累到足够的长度,能够揭示藻类物候的年际变化[26-27]。但是,对于高纬度和云层覆盖率高的地区,单一卫星传感器(如Landsat,其单颗卫星重访周期为16天)难以获得长时序数据。对于中小型湖泊,低空间分辨率的卫星数据(如MODIS,空间分辨率为250 m)易导致混合像元,使得藻类物候信息的提取十分困难[28]。这些限制性因素同样会导致水华的严重程度和物候监测的误差增加[29]。因此,通过多源卫星数据融合提高监测的时空分辨率,在一定程度上能减少不确定性因素的影响,从而提高物候监测的准确性。2014年,欧洲航天局(ESA)海洋颜色气候变化倡议(OC-CCI)项目基于SeaWiFS、MODIS和MERIS遥感数据,合并生成并验证了稳定的全球海洋颜色产品时间序列数据[30],这为研究海洋生态系统动态和藻类物候变化提供了坚实的数据基础。Acker等[31]利用SeaWiFS和MODIS数据描述了红海北部藻类的一般季节变化和物候特征。基于上述研究,Raitsos等[32]使用MODIS数据研究了藻类物候与红海环境条件的季节性关系。Demetriou等[33]基于23年(1997—2020年)的多源遥感数据(MODIS-Aqua、NOAA-20-VIIRS、NPP-VIIRS、Sentinel3A-OLCI)分析了塞浦路斯沿海水域藻类生物量和物候的季节性变化。此外,在大陆架边缘海域(斯科舍大陆架[34]、缅因湾[35]、巴伦支海、挪威海、北海[36]、白令海[37]、地中海[38]等)以及广大的中高纬度海域和低纬度热带海域都进行了大量的研究。
不同于海洋水色遥感,由于内陆水体的光学复杂性,通过卫星遥感对湖泊藻类物候进行定量估算和监测具有更大的挑战[39,40]。因此,目前仅在少数湖泊中使用遥感进行藻类物候的研究。Zhang等[41]运用10年的MERIS数据研究了波罗的海高度富营养化内陆水体藻类水华的空间变化,并且建立了物候(强度、时间和水华程度)的时间序列。Hu等[42]使用MODIS和Landsat数据,通过浮藻指数(FAI)研究了中国太湖蓝藻水华的时间变化,并通过阈值法来检测水华的存在,进一步确定了每个像素开始暴发水华的时间和持续时间。随后,洪恬林等[43]利用MODIS数据获取了长时间序列的Chl.a浓度数据,提取了藻类生长的物候指标,进一步分析了太湖不同湖区水华的时空分布特点和物候的年际变化及区域差异。此外,Matthews等[44]使用MERIS数据,通过研究南非50个湖泊的中位数Chl.a时间序列数据,确定了蓝藻水华区域范围及其生长趋势。Binding等[45]调查了美国和加拿大的森林湖泊每年的蓝藻水华、Chl.a浓度峰值的时间变化,研究了蓝藻的物候特征。值得注意的是,美国国家航空航天局(NASA)于2024年2月8日成功发射了新的地球观测卫星(浮游生物、气溶胶、云层和海洋生态系统,简称“PACE”),这将为浮游藻类物候遥感研究提供新的手段。综合分析这些遥感数据,研究者们在不同地区对藻类物候进行了监测和评估[46-48]。这些研究为深入了解海洋和内陆水体藻类物候特征提供了丰富而有力的数据支撑,也为未来遥感技术和数据分析方法的发展指明了方向。
表1用于监测藻类物候的遥感数据源
Tab.1 Remote sensing data sources for monitoring algal phenology
3 藻类遥感反演模型及物候指标
3.1 叶绿素a反演算法研究进展
Chl.a浓度常被用来表征藻类总生物量,也是藻类物候变化的关键因子[38-49]。因此,Chl.a浓度的季节性变化可以直接反映水体中藻类生物量和物候的变化规律。相关的研究多利用卫星反演的Chl.a产品来探索全球范围和特定区域内藻类物候的季节性和年际变化[33,50-51]。水体Chl.a浓度的遥感反演算法主要为经验类算法和分析类算法[52-53]。经验/半经验算法通常基于统计学方法以及光谱特征分析,而分析/半分析算法则多基于辐射传输方程推导得到。
经验类算法通过分析地面实测数据与对应遥感数据之间的相关关系,针对某一特定波段或不同波段的函数组合,实现Chl.a浓度估算[54]。常用的经验方法有单波段模型、波段比值模型等。单波段模型主要是构建Chl.a浓度与最优波段反射率之间的相关性方程。虽然单波段模型在一些湖泊的反演效果较好[55],但其普适性和迁移性较差[56]。波段比值模型通过建立两个特定波段比值与Chl.a浓度之间的关系,如蓝绿波段比值法,被广泛应用于大洋等Ⅰ类水体中[57]。波段比值法能够有效减少悬浮物、黄色物质等污染物以及大气的干扰,从而提高反演精度,因此在国内外得到广泛应用。Le等[58]基于MERIS数据建立了双波段比值拟合模型。乐成峰等[59]根据季节变化采用多种方法对太湖Chl.a浓度进行了反演,结果表明波段比值法在春季和秋季是最佳的反演算法,能够消除部分环境影响。由于受到有色可溶性有机物(CDOM)和非藻类颗粒物的影响,在内陆和近岸水体中红波段与近红外波段算法表现更好[57]。此外还有光谱微分法、非线性最优化法等。经验类方法虽然简单易于实现,但是其迁移性和鲁棒性较差,在实际运用中需要根据情况重新获取最佳的反演波段。
半分析方法将理论分析和经验统计分析相结合,通过已知水质参数的光谱特征选择最佳的波段或波段组合来估算Chl.a浓度,主要代表包括三波段法、四波段等。三波段法引入第三波段以消除其他光学活性成分(SPM和CDOM)对Chl.a浓度反演的影响[60]。四波段法是在三波段模型的基础再引入一个波段以减弱纯水和悬浮物的干扰[61]。毕顺等[62]利用OLCI数据对洱海Chl.a浓度进行反演,发现三波段模型适用性较高。Lv等[63]比较了5个典型内陆湖泊中的4种Chl.a反演算法,结果显示四波段算法在常规水体反演中表现最佳。综合来看,半分析方法具有较广泛的适用性和较高的精度,可应用于不同类型水体的Chl.a浓度反演。
传统的经验模型虽然结构简单且易于实现,但普适性和鲁棒性较差。半分析模型的计算过程相对复杂,在处理复杂多变的水体时受到很大的限制。相比之下,机器学习可以利用复杂的网络和结构来捕获输入数据的非线性特征,被广泛用于海洋、沿海和内陆水域环境的研究[64-65]。目前主流的机器学习算法包括神经网络(ANN)[66]、极端梯度提升树(BST)[67]、支持向量机(SVM)[68]和随机森林(RF)[69]等。
3.2 浮游藻类物候指标
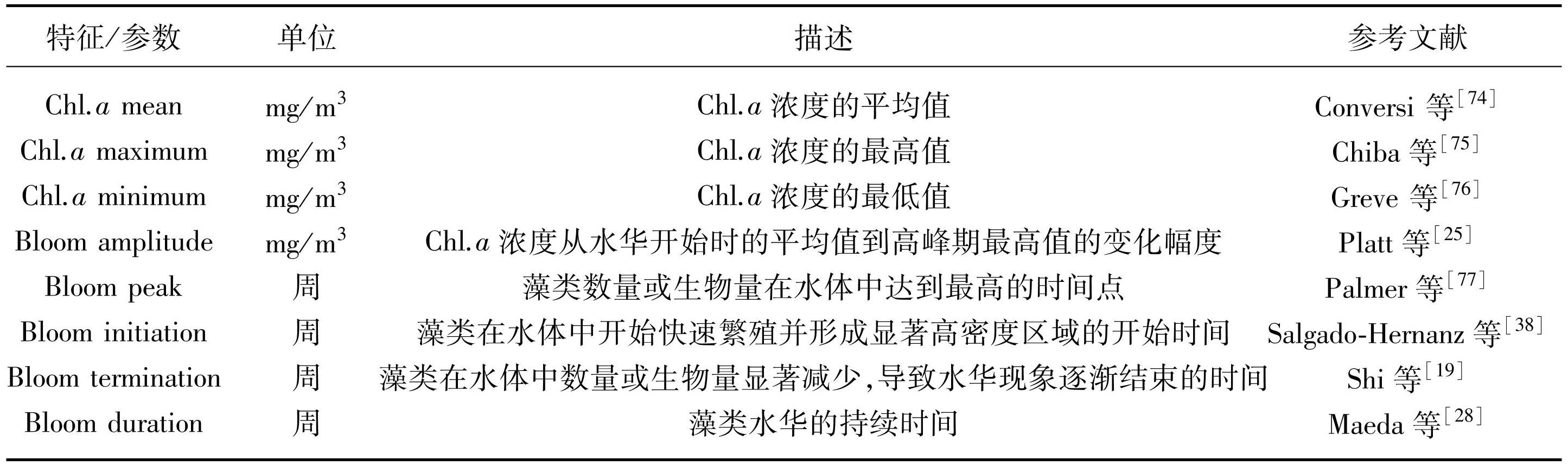
水体生态系统中的浮游藻类,对环境和气候的变化表现出了高度的敏感性[70]。在气候变暖的背景下,藻类大量繁殖形成水华的频率和强度增加[71]。相较于陆地植被,藻类的生命周期较短,这意味着它们能够更快速地对气候和环境的波动做出响应。研究者们通过对现场和实验室数据的观测以及模型的建构,已经证实了藻类物候对于如温度、养分以及其他环境变量的变化所表现出的高度敏感性[72-73]。当环境条件发生变化时,藻类物候也会相应地发生调整。多种特征被广泛应用于定量化藻类物候,这些典型的物候包括:起始时间、峰值时间、终止时间、持续时间、浮游藻类Chl.a浓度的振幅等(表2)。
表2藻类物候的时间指标
Tab.2 Temporal indicators of algal phenology
4 物候遥感提取的技术方法
藻类物候遥感提取主要利用Chl.a浓度时间序列数据,能够呈现出藻类生长的季节和年际变化规律。遥感数据在采集的过程中会受到云、气溶胶、水汽、太阳高度角、传感器本身性能以及观测条件差异等因素的影响,使Chl.a浓度时序数据曲线中存在大量的噪声(异常值和缺失值),无法直接提取物候信息及趋势分析。因此,需要对Chl.a时序数据进行重构和空值插补,以达到数据连续、平滑和降噪。重构后的时序数据再利用数学模型提取藻类生长过程中的关键时间点,从而实现物候的获取(图2)。
4.1 Chl.a遥感时序数据预处理方法
遥感数据的时间序列曲线重构方法总体上可分为滤波和拟合两类(表3)。滤波是一种信号处理技术,通常是指对输入的时序数据通过数学变换,将高频部分噪声利用低通滤波器进行去噪的过程,以消除噪声、平滑信号或突出特定频率范围信号的方法。常用的滤波方法包括Savitzky-Golay滤波法、滑动中值/均值滤波法、迭代滤波法、傅里叶变换低通滤波法、小波变换滤波法、高斯滤波、Lanczos滤波等。如Zoljoodi等[13]使用Savitzky-Golay滤波器对Chl.a的时间序列信号进行平滑处理,并且使用数据插值经验正交函数来填补遥感数据集中的时间和空间空白。Vantrepotte等[78]使用迭代滤波算法来分析Chl.a的年际变化。Winder等[79]使用小波分析方法提取藻类生物量的时间序列数据,并分析其主要变化周期。Demetriou等[33]使用高斯滤波器平滑了Chl.a浓度累积总和的异常值,并用于识别所需的物候阶段。Salgado-Hernanz等[38]使用线性插值来填补数据中的空白,并使用平均迭代滤波方法对数据进行平滑处理。
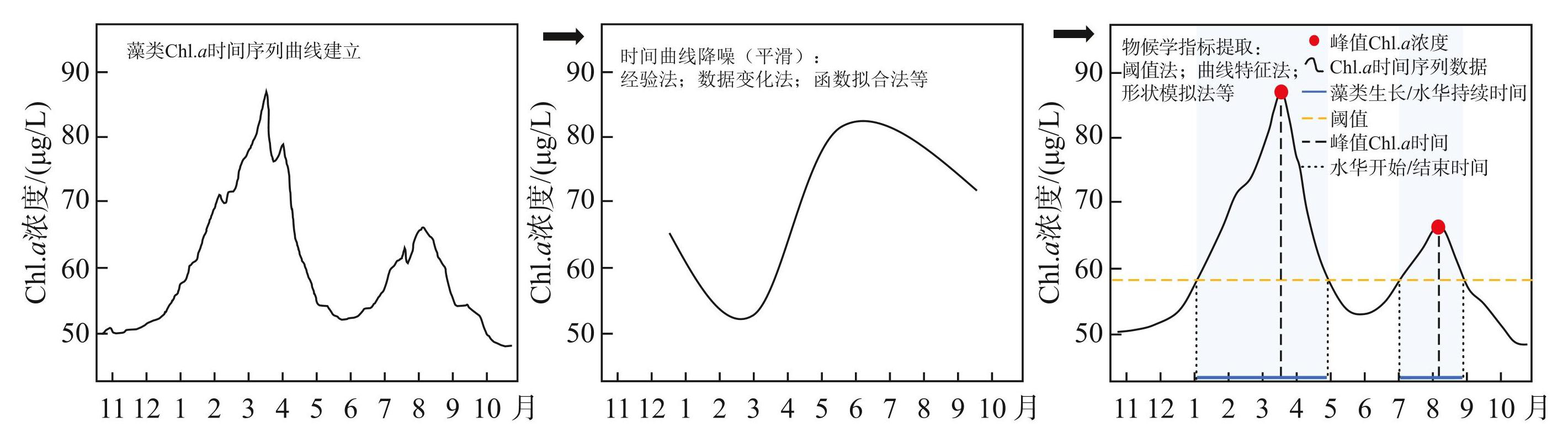
图2藻类长时序数据处理及物候参数提取流程
Fig.2Processing long-term algal data and extracting phenological parameters
表3藻类物候遥感中常用的数据预处理方法
Tab.3 Common data preprocessing methods in remote sensing of algal phenology

拟合法通过某种函数(如傅里叶级数、非对称高斯函数、分段逻辑函数、双Logistic函数等)对时序数据曲线进行最小二乘拟合,用拟合得到的平滑曲线代替原来的时序数据曲线来实现平滑去噪。由于拟合法不需要事先定义阈值或窗口大小,其操作更加方便简单,近年来被广泛应用于时序数据处理中。如Kostadinov等[80]通过减去数据拟合的最小二乘线,使用离散傅里叶变换(discrete fourier transform)将时间序列转换为频域,消除可能的长期趋势以确保正确的时序数据。Palmer 等[77]比较了双逻辑曲线拟合、非对称高斯函数和Savitzky-Golay滤波3种时间序列平滑方法,并与原位Chl.a测得的物候进行比较,探讨方法上的局限性和物候指标之间的相关性。Platt等[25]使用非线性最小二乘法拟合了SeaWiFS叶绿素数据。Maeda等[28]使用局部多项式回归拟合(LOESS)方法进行时间序列平滑处理,该方法对于去除高频噪声和优化物候指标的计算十分有效。此外Grossi等[14]使用5天居中移动均值来平滑Chl.a时间序列数据。
4.2 物候期参数提取方法
利用遥感数据对藻类物候特征进行分析提取时,除了重构遥感数据的时间序列曲线以外,还需要进行物候信息的提取。遥感物候期时间节点基于Chl.a浓度时序数据的藻类生长曲线形态,通过设定一定的阈值或者寻找曲线变化速率的极值点来确定。藻类物候信息提取方法可分为阈值法、曲线特征法和数学分析法3大类。
4.2.1 阈值法
阈值法主要是通过设置遥感时序数据(Chl.a浓度)的阈值来确定藻类生长开始和结束的日期。因此,确定藻类大量繁殖或将不同程度水华分开的阈值是建立物候识别和水华监测的关键步骤。阈值标准能直接从遥感叶绿素时间序列估计中得出[12,81-82],亦或将函数拟合到叶绿素时间序列后计算得到[34,47],亦或通过叶绿素浓度的累积求和计算得到[83],包括绝对阈值法、相对阈值法、最大斜率阈值法、累计阈值法等。在全球藻类物候研究中,绝对阈值法因简单易操作,是早期藻类候遥感中常用的方法,但由于不同的水体环境及藻类生长的差异性,绝对阈值法存在相当大的局限性。相对阈值法因能适应藻类生长过程中的巨大变化,适用性较强。无论哪种阈值方法的选择都与藻类季节性周期的形态变化密切关联。阈值法通常以高于藻类Chl.a浓度中值的1%~30%作为阈值来计算物候时间节点,该范围内不同阈值计算出的物候指标差异较小,常用5%作为阈值[82]。Henson等[81]在伊尔明厄海的研究中也采用5%的阈值,证明了在区域尺度上,混合层中藻类生长的起始时间与物候之间存在着一定的关系。Thomalla等[84]同样采用5%的阈值研究了南大洋藻类生物量季节性循环的区域特征。虽然不同的方法和阈值标准可能产生相似的结果,但方法和阈值的选择需要仔细检查相应的藻类季节性的周期变化[46]。
4.2.2 曲线特征法
曲线特征法是通过分析时序曲线中具有特定变化特征的点(拐点、极值点等),利用曲线的曲率变化和导数拟合得到数据曲线进行分析,根据其变化特征来获取藻类物候变化特征,常用的方法包括估计拐点、曲率变化极值法、拟合密度函数和广义线性模型等[85-86]。Rolinski等[87]通过估计拐点、拟合Weibull型函数和线性拟合数值来确定春季水华发育的开始、最大和结束时间。Mao等[88]通过研究正弦曲线与Chl.a时间序列数据的相关性,根据遥感数据得到平均Chl.a浓度长时序曲线形状,使用非线性拟合函数来描述藻类的季节变化。Vargas等[85]通过SeaWiFS叶绿素浓度的广义线性模型(GLM)提取了北大西洋藻类物候,使用最小二乘拟合方法提取了水华的开始和结束日期,并与其他研究者观察到的藻类生物量年际变化的曲线形状和时间基本一致。Friedland等[10]则使用变化点统计方法,分析季节性藻类的繁殖趋势,从而深入研究这些生物在不同季节的生长模式及其对环境变化的响应。
4.2.3 数学分析法
数学分析法通过数学模型或数学变换手段从遥感时序数据中提取藻类的物候信息,并在藻类物候研究中发挥重要作用。高斯分布常被用于分析时间序列Chl.a浓度数据,如Zhai等[34]通过高斯分布分析加的斯湾浮藻类大量繁殖的规模和持续时间。基于高斯模型算法的优点在于能够确定时序曲线周期形状和水华特性的其他关键参数,对单峰、双峰水华的识别和物候的监测更加准确[89]。Palacz等[90]利用Hilbert-Huang变换对卫星产品进行分析,用于平滑Chl.a数据,发现南海Chl.a浓度总体呈增加趋势。Zhang等[91]使用Holo-Hilbert光谱分析来检查藻类水华的时间和幅度趋势。在研究藻类季节变化的过程中,也常利用多维经验模态分解来分析Chl.a趋势的时空演化[92]。
5 浮游藻类物候变化的影响因素
5.1 气候要素及其变化对浮游藻类物候的影响
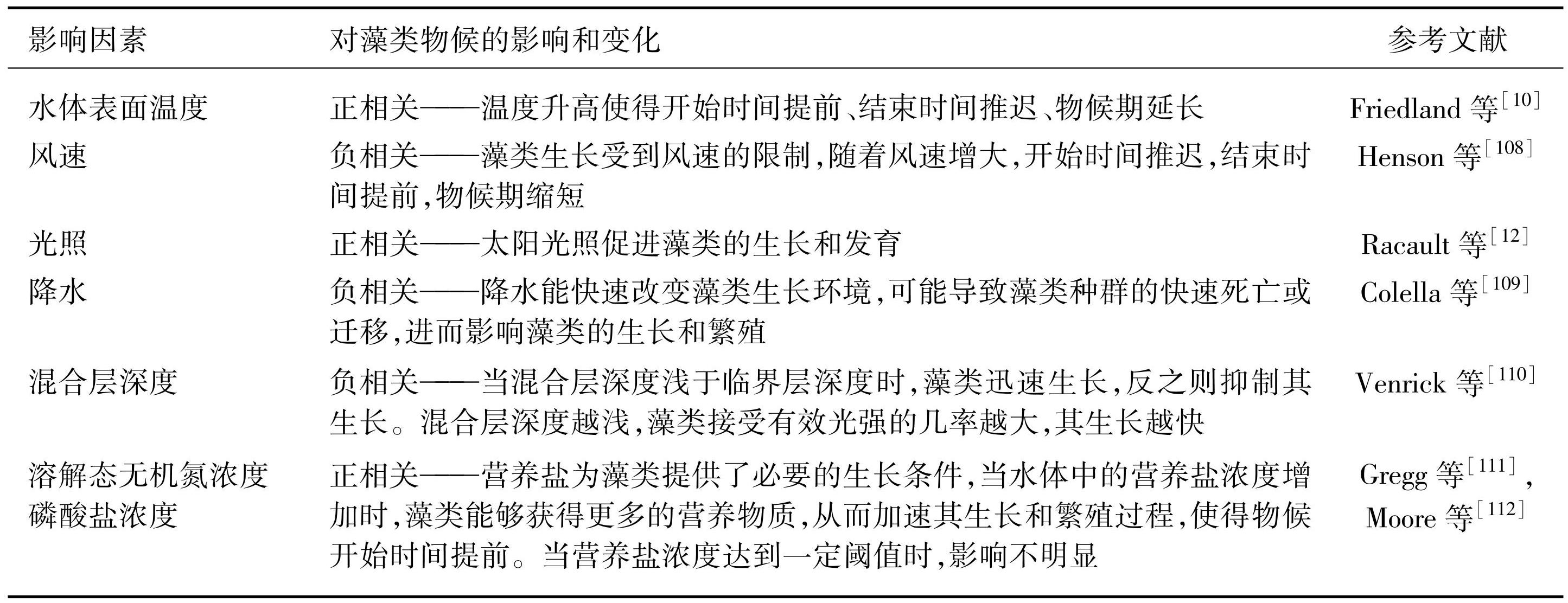
近年来气候变化对藻类物候具有显著影响。无论海洋生态系统还是内陆水体生态系统,全球范围的物候期都有明显的提前趋势,表现出藻类生长季开始提早、结束推迟和生长季延长的趋势。藻类生长周期呈现出随纬度变化的趋势:在高纬度地区,藻类生长多为单次季节性暴发;中纬度海域的浮游藻类物候表现出明显的春季和秋季两次暴发;对于低纬度海域而言,藻类生长受到短期气候变化等其他复杂因素的影响[93]。在其他自然气候因素方面,藻类快速生长的开始与日照和水体分层的季节性有关,受到温度、营养盐、降水、风速等因素的影响[19,94]。Wernand等[95]发现全球Chl.a浓度并未增加或减少,这表明Chl.a浓度的变化可能受到多种因素的影响,而不仅仅是时间的推移。在海洋学和气候因素中,如水温、太阳辐射、水体分层、水混合、营养盐浓度、沿海上升流、风力强迫、大气沉降和二氧化碳流入都是影响藻类生物量季节性周期变化的因素(表4),而驱动每个区域物候指标变异性的潜在机制可能又是复杂的[96]。在开阔的海洋中,夏季太阳加热以及冬季冷却表面混合层和强风引起的对流过程是藻类Chl.a浓度季节性变化的主要驱动力[97-100]。海面风应力是物理海洋学中的一个重要因素,因为风应力不仅推动海洋的环流,还主导着表面波场的形成和风驱动的海洋表面洋流的生成[101-103]。因此,通过对风应力的研究,可以更好地理解其对藻类水华物候动态的潜在影响。此外,Wasmund[104]指出,水柱中藻类的光穿透深度和垂直分布是决定夏季水华形成的重要因素。藻类的生存和繁殖模式在极地、热带/亚热带和沿海地区有显著差异,除了上述提到的因素外,湖/海冰、潮汐混合以及淡水径流等都会影响藻类生长所需光/养分的可用性和季节性分布[105]。
在全球范围内,藻类的生长条件不仅受到大尺度物理强迫和区域季节变化的影响,还受到气候模式的驱动。这些气候模式指数,如厄尔尼诺南方涛动指数、北大西洋涛动和南方环流模式等都与藻类的物候密切相关[106-107]。因此,气候的变化往往会直接影响浮游藻类的物候变化,而这些因素又相互关联并受到极端气候模式的影响。
表4环境变量和气候指数指标摘要
Tab.4 Summary of environmental variables and climate index indicators
5.2 城市化和人类活动对浮游藻类物候的影响
藻类物候的变化同样也受城市化和人类活动的复杂影响,这种影响受多种因素的干预调节。具体来说,不仅包括环境中的变化,还涉及到地表微量营养素的消耗以及人类生产和生活所带来的直接影响[113]。随着城市化的进行,养分通过河流连接进入城市周围水体或近海岸区域[114]。此外,城市化导致的水温升高也可能与此有关,快速城市化引起地表温度的持续增加可能会使城市周围的湖泊或流入河流的水温升高[115-116],使藻类生长期提前、结束期推迟。同时,城市扩张还能通过改变湖陆微风环流来提高郊区风速,从而在一定程度上改变藻类的物候特征[117]。
人类活动对藻类水华的形成和演变产生了深远的影响,尤其是水体的富营养化。研究表明,农业生产中的土地利用变化对湖泊藻类的物候产生了显著影响[4,118-119]。这些变化可能会导致水体中氮磷比率(N∶P)的失衡,从而进一步促进藻类大量繁殖的发生[120]。这一理论后来被数值模型的研究所证实,表明N∶P比率在调控水华发展和藻类物候中的关键作用[121-122]。其他因素,如表面硝酸盐和硅酸盐的消耗,也会在一定程度上影响藻类生长的时间[123-124]。近期研究已经记录并预测了城市化对浮游植物丰度以及物候的潜在影响[114,125]。上述研究结果表明,城市化可以通过改变浮游藻类的关键驱动因素来影响藻类水华的严重程度和物候。因此,阐明人类活动和气候变化对富营养化水体中藻类物候的交互作用,对于提高藻类物候预测和水华管理的能力至关重要。
6 结论与展望
本文从多个角度系统总结了藻类物候遥感技术、监测方法,以及多源遥感影像在该领域的应用研究,同时探讨了其发展趋势和取得的成果。进一步分析了浮游藻类物候变化的影响因素,并对藻类物候特性趋势及可能影响年际变化的潜在驱动因素进行了深入的梳理和分析。当前藻类物候的遥感监测研究还处于不断发展和完善的阶段,尽管现有的研究已经取得了一些进展,但也面临着许多问题和挑战,包括Chl.a浓度遥感反演算法的准确性和普适性、多源遥感数据融合的差异性、长时间序列遥感监测的可行性以及全球气候变化对水体藻类生长的影响。此外,目前藻类物候研究主要集中在单一指标的监测和估计,对于复杂水体和不同藻类类型的适应性还有待进一步深入研究。不同遥感数据源的选择、大气校正以及模型验证等方面的问题仍然是需要关注并解决的难题。
未来,藻类物候遥感的发展方向可概括为以下几个方面:(1)物候指标选择的优化:提升Chl.a浓度遥感反演算法的精确性和普适性,考虑藻类物候研究向多指标观测发展,以弥补仅依赖单指标(Chl.a浓度)的局限性;(2)物候识别算法的改进和发展:坚持藻类固有光学特性的机理研究,探讨藻类光学性质与其物候的关系;(3)在应用方面,建立和完善长时间序列的遥感监测数据库,以支持藻类物候的长期趋势分析和全球变化影响评估,实时监测水体中藻类生长繁殖状况,从而构建综合的水体风险因子评级体系,为决策者提供关于水体管理、保护和恢复的重要信息,帮助实现水体健康和可持续利用。

图1(a)以“Phytoplankton”“Remote Sensing”“Phenology”为主题词在Web of science中检索得到的可视化图(关键词越大表示出现频率越高);(b)浮游藻类物候研究发文和引文数量的变化趋势
Fig.1(a) A visualization map retrieved from Web of Science using “Phytoplankton”, “Remote Sensing” and “Phenology” as keywords(the larger the keyword, the higher the frequency of occurrence);(b) Trends in publications and citations on phytoplankton phenology research
下载:
全尺寸图片

图2藻类长时序数据处理及物候参数提取流程
Fig.2Processing long-term algal data and extracting phenological parameters
下载:
全尺寸图片




| [1] |
Edwards M, Richardson AJ. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature,2004,430:881-884. DOI:10.1038/nature02808.
|
| [2] |
Di Lorenzo E, Ohman MD. A double-integration hypothesis to explain ocean ecosystem response to climate forcing. Proceedings of the National Academy of Sciences of the United States of America,2013,110(7):2496-2499. DOI:10.1073/pnas.1218022110.
|
| [3] |
Niu Y, Liu CL, Lu XL et al. Phytoplankton blooms and its influencing environmental factors in the southern Yellow Sea. Regional Studies in Marine Science,2021,47:101916. DOI:10.1016/j.rsma.2021.101916.
|
| [4] |
Boyd PW, Lennartz ST, Glover DM et al. Biological ramifications of climate-change-mediated oceanic multi-stressors. Nature Climate Change,2015,5:71-79. DOI:10.1038/nclimate2441.
|
| [5] |
Behrenfeld MJ, Boss ES. Student's tutorial on bloom hypotheses in the context of phytoplankton annual cycles. Global Change Biology,2018,24(1):55-77. DOI:10.1111/gcb.13858.
|
| [6] |
Platt T, Sathyendranath S. Ecological indicators for the pelagic zone of the ocean from remote sensing. Remote Sensing of Environment,2008,112(8):3426-3436. DOI:10.1016/j.rse.2007.10.016.
|
| [7] |
Gittings JA, Raitsos DE, Brewin RJW et al. Links between phenology of large phytoplankton and fisheries in the northern and central red sea. Remote Sensing,2021,13(2):231. DOI:10.3390/rs13020231.
|
| [8] |
Sverdrup HU. On conditions for the vernal blooming of phytoplankton. ICES Journal of Marine Science,1953,18(3):287-295. DOI:10.1093/icesjms/18.3.287.
|
| [9] |
Zhao H. Satellite perspective on phytoplankton bloom phenology and linkages with changing environments in the northern high-latitude oceans. Indiana State University,2023.
|
| [10] |
Friedland KD, Mouw CB, Asch RG et al. Phenology and time series trends of the dominant seasonal phytoplankton bloom across global scales. Global Ecology and Biogeography,2018,27(5):551-569. DOI:10.1111/geb.12717.
|
| [11] |
Groetsch PMM, Simis SGH, Eleveld MA et al. Spring blooms in the Baltic Sea have weakened but lengthened from 2000 to 2014. Biogeosciences,2016,13(17):4959-4973. DOI:10.5194/bg-13-4959-2016.
|
| [12] |
Racault MF, Le Quéré C, Buitenhuis E et al. Phytoplankton phenology in the global ocean. Ecological Indicators,2012,14(1):152-163. DOI:10.1016/j.ecolind.2011.07.010.
|
| [13] |
Zoljoodi M, Moradi M, Moradi N. Seasonal and interannual cycles of total phytoplankton phenology metrics in the Persian Gulf using ocean color remote sensing. Continental Shelf Research,2022,237:104685. DOI:10.1016/j.csr.2022.104685.
|
| [14] |
Grossi F, Lagasio M, Napoli A et al. Phytoplankton spring bloom in the NW Mediterranean Sea under climate change. Science of the Total Environment,2024,914:169884. DOI:10.1016/j.scitotenv.2024.169884.
|
| [15] |
Xu H, Paerl HW, Qin BQ et al. Determining critical nutrient thresholds needed to control harmful cyanobacterial blooms in eutrophic Lake Taihu, China. Environmental Science & Technology,2015,49(2):1051-1059. DOI:10.1021/es503744q.
|
| [16] |
Santos M, Mouriño H, Moita MT et al. Characterizing phytoplankton biomass seasonal cycles in two NE Atlantic coastal bays. Continental Shelf Research,2020,207:104200. DOI:10.1016/j.csr.2020.104200.
|
| [17] |
Cleveland JS. Regional models for phytoplankton absorption as a function of chlorophyll a concentration. Journal of Geophysical Research: Oceans,1995,100(C7):13333-13344. DOI:10.1029/95JC00532.
|
| [18] |
Schalles JF. Optical remote sensing techniques to estimate phytoplankton chlorophyll a concentrations in coastal. Remote Sensing and Digital Image Processing. Dordrecht: Springer Netherlands,2006:27-79. DOI:10.1007/1-4020-3968-9_3.
|
| [19] |
Shi K, Zhang YL, Zhang YB et al. Phenology of phytoplankton blooms in a trophic lake observed from long-term MODIS data. Environmental Science & Technology,2019,53(5):2324-2331. DOI:10.1021/acs.est.8b06887.
|
| [20] |
McClain CR. A decade of satellite ocean color observations. Annual Review of Marine Science,2009,1:19-42. DOI:10.1146/annurev.marine.010908.163650.
|
| [21] |
Woods T, Kaz A, Giam X. Phenology in freshwaters: A review and recommendations for future research. Ecography,2022,2022(6):e05564. DOI:10.1111/ecog.05564.
|
| [22] |
Bajocco S, Raparelli E, Teofili T et al. Text mining in remotely sensed phenology studies: A review on research development,main topics,and emerging issues. Remote Sensing,2019,11(23):2751. DOI:10.3390/rs11232751.
|
| [23] |
Dash J, Jones M, Nightingale J. Validating satellite-derived vegetation phenology products: Second international workshop on the validation of satellite-based land surface phenology products. Wisconsin: Wiley Online Library,2013.
|
| [24] |
Platt T, Sathyendranath S, White GN et al. Diagnostic properties of phytoplankton time series from remote sensing. Estuaries and Coasts,2010,33(2):428-439. DOI:10.1007/s12237-009-9161-0.
|
| [25] |
Platt T, White GN III, Zhai L et al. The phenology of phytoplankton blooms: Ecosystem indicators from remote sensing. Ecological Modelling,2009,220(21):3057-3069. DOI:10.1016/j.ecolmodel.2008.11.022.
|
| [26] |
Ganguly S, Friedl MA, Tan B et al. Land surface phenology from MODIS: Characterization of the Collection 5 global land cover dynamics product. Remote Sensing of Environment,2010,114(8):1805-1816. DOI:10.1016/j.rse.2010.04.005.
|
| [27] |
Hou XJ, Feng L, Dai YH et al. Global mapping reveals increase in lacustrine algal blooms over the past decade. Nature Geoscience,2022,15:130-134. DOI:10.1038/s41561-021-00887-x.
|
| [28] |
Maeda EE, Lisboa F, Kaikkonen L et al. Temporal patterns of phytoplankton phenology across high latitude lakes unveiled by long-term time series of satellite data. Remote Sensing of Environment,2019,221:609-620. DOI:10.1016/j.rse.2018.12.006.
|
| [29] |
Zhang YC, Shi K, Cao Z et al. Effects of satellite temporal resolutions on the remote derivation of trends in phytoplankton blooms in inland waters. ISPRS Journal of Photogrammetry and Remote Sensing,2022,191:188-202. DOI:10.1016/j.isprsjprs.2022.07.017.
|
| [30] |
Hollmann R, Merchant CJ, Saunders R et al. The ESA climate change initiative: Satellite data records for essential climate variables. Bulletin of the American Meteorological Society,2013,94(10):1541-1552. DOI:10.1175/bams-d-11-00254.1.
|
| [31] |
Acker J, Leptoukh G, Shen S et al. Remotely-sensed chlorophyll a observations of the northern Red Sea indicate seasonal variability and influence of coastal reefs. Journal of Marine Systems,2008,69(3/4):191-204. DOI:10.1016/j.jmarsys.2005.12.006.
|
| [32] |
Raitsos DE, Pradhan Y, Brewin RJW et al. Remote sensing the phytoplankton seasonal succession of the Red Sea. PLoS One,2013,8(6):e64909. DOI:10.1371/journal.pone.0064909.
|
| [33] |
Demetriou M, Raitsos DE, Kournopoulou A et al. Phytoplankton phenology in the coastal zone of Cyprus,based on remote sensing and in situ observations. Remote Sensing,2021,14(1):12. DOI:10.3390/rs14010012.
|
| [34] |
Zhai L, Platt T, Tang C et al. Phytoplankton phenology on the Scotian shelf. ICES Journal of Marine Science,2011,68(4):781-791. DOI:10.1093/icesjms/fsq175.
|
| [35] |
Record NR, Balch WM, Stamieszkin K. Century-scale changes in phytoplankton phenology in the Gulf of Maine. PeerJ,2019,7:e6735. DOI:10.7717/peerj.6735.
|
| [36] |
Silva E, Counillon F, Brajard J et al. Twenty-one years of phytoplankton bloom phenology in the Barents, Norwegian,and North Seas. Frontiers in Marine Science,2021,8:746327. DOI:10.3389/fmars.2021.746327.
|
| [37] |
Nielsen JM, Sigler MF, Eisner LB et al. Spring phytoplankton bloom phenology during recent climate warming on the Bering Sea shelf. Progress in Oceanography,2024,220:103176. DOI:10.1016/j.pocean.2023.103176.
|
| [38] |
Salgado-Hernanz PM, Racault MF, Font-Muñoz JS et al. Trends in phytoplankton phenology in the Mediterranean Sea based on ocean-colour remote sensing. Remote Sensing of Environment,2019,221:50-64. DOI:10.1016/j.rse.2018.10.036.
|
| [39] |
Fang C, Song KS, Paerl HW et al. Global divergent trends of algal blooms detected by satellite during 1982-2018. Global Change Biology,2022,28(7):2327-2340. DOI:10.1111/gcb.16077.
|
| [40] |
Du YX, Song KS, Liu G. Monitoring optical variability in complex inland waters using satellite remote sensing data. Remote Sensing,2022,14(8):1910. DOI:10.3390/rs14081910.
|
| [41] |
Zhang DX, Lavender S, Muller JP et al. MERIS observations of phytoplankton phenology in the Baltic Sea. Science of the Total Environment,2018,642:447-462. DOI:10.1016/j.scitotenv.2018.06.019.
|
| [42] |
Hu CM, Lee ZP, Ma RH et al. Moderate resolution imaging spectroradiometer(MODIS)observations of cyanobacteria blooms in Taihu Lake, China. Journal of Geophysical Research: Oceans,2010,115(C4): C04002. DOI:10.1029/2009JC005511.
|
| [43] |
Hong TL, Li YM, Lv H et al. Patterns of phytoplankton phenology and its response to temperature of water surface in Lake Taihu based on MODIS data. Journal of Geo-information Science,2020,22(10):1935-1945. DOI:10.12082/dqxxkx.2020.200206.[洪恬林, 李云梅, 吕恒等. 基于MODIS数据的太湖浮游植物物候变化及其对水表温度的响应. 地球信息科学学报,2020,22(10):1935-1945.]
|
| [44] |
Matthews MW, Bernard S, Robertson L. An algorithm for detecting trophic status(chlorophyll-a),cyanobacterial-dominance,surface scums and floating vegetation in inland and coastal waters. Remote Sensing of Environment,2012,124:637-652. DOI:10.1016/j.rse.2012.05.032.
|
| [45] |
Binding CE, Greenberg TA, Bukata RP. Time series analysis of algal bloomsin lake of the woods using the MERIS maximum chlorophyll index. Journal of Plankton Research,2011,33(12):1847-1852. DOI:10.1093/plankt/fbr079.
|
| [46] |
Cole H, Henson S, Martin A et al. Mind the gap: The impact of missing data on the calculation of phytoplankton phenology metrics. Journal of Geophysical Research: Oceans,2012,117(C8): C08030. DOI:10.1029/2012JC008249.
|
| [47] |
González Taboada F, Anadón R. Seasonality of North Atlantic phytoplankton from space: Impact of environmental forcing on a changing phenology(1998-2012). Global Change Biology,2014,20(3):698-712. DOI:10.1111/gcb.12352.
|
| [48] |
Kahru M, Brotas V, Manzano-Sarabia M et al. Are phytoplankton blooms occurring earlier in the Arctic?Global Change Biology,2011,17(4):1733-1739. DOI:10.1111/j.1365-2486.2010.02312.x.
|
| [49] |
Taboada FG, Barton AD, Stock CA et al. Seasonal to interannual predictability of oceanic net primary production inferred from satellite observations. Progress in Oceanography,2019,170:28-39. DOI:10.1016/j.pocean.2018.10.010.
|
| [50] |
Park J, Kim JH, Kim HC et al. Environmental forcings on the remotely sensed phytoplankton bloom phenology in the central Ross Sea polynya. Journal of Geophysical Research: Oceans,2019,124(8):5400-5417. DOI:10.1029/2019JC015222.
|
| [51] |
Kheireddine M, Mayot N, Ouhssain M et al. Regionalization of the red sea based on phytoplankton phenology: A satellite analysis. Journal of Geophysical Research: Oceans,2021,126(10):e2021JC017486. DOI:10.1029/2021JC017486.
|
| [52] |
McClain CR, Signorini SR, Christian JR. Subtropical gyre variability observed by ocean-color satellites. Deep Sea Research Part II: Topical Studies in Oceanography,2004,51(1/2/3):281-301. DOI:10.1016/j.dsr2.2003.08.002.
|
| [53] |
Feng L, Hu CM, Chen XL et al. Assessment of inundation changes of Poyang Lake using MODIS observations between 2000 and 2010. Remote Sensing of Environment,2012,121:80-92. DOI:10.1016/j.rse.2012.01.014.
|
| [54] |
房冲. 国际界湖水质遥感反演及时空演变分析[学位论文]. 北京: 中国科学院大学,2020.
|
| [55] |
Zhang DX, Ruan RZ, Yan MC et al. Spatial distribution of chlorophyll a in Chaohu Lake based on MODIS. Geospatial Information,2012,10(5):64-66,92.[张道祥, 阮仁宗, 颜梅春等. 基于MODIS的巢湖叶绿素a空间分布. 地理空间信息,2012,10(5):64-66,92.]
|
| [56] |
Meng FX, Cheng XB, Zhang GL et al. Quantitative inversion of nearshore suspended sediment and chlorophyll-a concentration based on Landsat-8 data in South China Sea. Global Geology,2017,36(2):616-623,642.[孟凡晓, 陈圣波, 张国亮等. 基于Landsat-8数据南海近岸悬浮泥沙与叶绿素a浓度定量反演. 世界地质,2017,36(2):616-623,642.]
|
| [57] |
Luo JCY, Qin LJ, Mao P et al. Research progress in the retrieval algorithms for chlorophyll-a,a key element of water quality monitoring by remote sensing. Remote Sensing Technology and Application,2021,36(3):473-488.[罗婕纯一, 秦龙君, 毛鹏等. 水质遥感监测的关键要素叶绿素a的反演算法研究进展. 遥感技术与应用,2021,36(3):473-488.]
|
| [58] |
Le CF, Hu CM, Cannizzaro J et al. Evaluation of chlorophyll-a remote sensing algorithms for an optically complex estuary. Remote Sensing of Environment,2013,129:75-89. DOI:10.1016/j.rse.2012.11.001.
|
| [59] |
Le CF, Li YM, Sun DY et al. Research on chlorophyll concentration retrieval models of Taihu Lake based on seasonal difference. National Remote Sensing Bulletin,2007,11(4):473-480.[乐成峰, 李云梅, 孙德勇等. 基于季节分异的太湖叶绿素浓度反演模型研究. 遥感学报,2007,11(4):473-480.]
|
| [60] |
Gitelson AA, Dall'Olmo G, Moses W et al. A simple semi-analytical model for remote estimation of chlorophyll-a in turbid waters: Validation. Remote Sensing of Environment,2008,112(9):3582-3593. DOI:10.1016/j.rse.2008.04.015.
|
| [61] |
Le CF, Li YM, Zha Y et al. A four-band semi-analytical model for estimating chlorophyll a in highly turbid lakes: The case of Taihu Lake, China. Remote Sensing of Environment,2009,113(6):1175-1182. DOI:10.1016/j.rse.2009.02.005.
|
| [62] |
Bi S, Li YM, Lv H et al. Estimation of chlorophyll-a concentration in Lake Erhai based on OLCI data. J Lake Sci,2018,30(3):701-12. DOI:10.18307/2018.0312.[毕顺, 李云梅, 吕恒等. 基于OLCI数据的洱海叶绿素a浓度估算. 湖泊科学,2018,30(3):701-12.]
|
| [63] |
Lv H, Li XJ, Wang YN et al. Evaluation of chlorophyll-a retrieval algorithms based on MERIS bands for optically varying eutrophic inland lakes. Science of the Total Environment,2015,530/531:373-382. DOI:10.1016/j.scitotenv.2015.05.115.
|
| [64] |
Fang C, Song CC, Wen ZD et al. A novel chlorophyll-a retrieval model based on suspended particulate matter classification and different machine learning. Environmental Research,2024,240(Pt 1):117430. DOI:10.1016/j.envres.2023.117430.
|
| [65] |
Cao ZG, Shen M, Kutser T et al. What water color parameters could be mapped using MODIS land reflectance products: A global evaluation over coastal and inland waters. Earth-Science Reviews,2022,232:104154. DOI:10.1016/j.earscirev.2022.104154.
|
| [66] |
Pahlevan N, Smith B, Binding CR et al. Hyperspectral retrievals of phytoplankton absorption and chlorophyll-a in inland and nearshore coastal waters. Remote Sensing of Environment,2021,253:112200. DOI:10.1016/j.rse.2020.112200.
|
| [67] |
Cao ZG, Ma RH, Duan HT et al. A machine learning approach to estimate chlorophyll-a from Landsat-8 measurements in inland lakes. Remote Sensing of Environment,2020,248:111974. DOI:10.1016/j.rse.2020.111974.
|
| [68] |
Sun DY, Li YM, Wang Q. A unified model for remotely estimating chlorophyll a in Lake Taihu, China,based on SVM and in situ hyperspectral data. IEEE Transactions on Geoscience and Remote Sensing,2009,47(8):2957-2965. DOI:10.1109/TGRS.2009.2014688.
|
| [69] |
Hafeez S, Wong MS, Ho HC et al. Comparison of machine learning algorithms for retrieval of water quality indicators in case-Ⅱ waters: A case study of Hong Kong. Remote Sensing,2019,11(6):617. DOI:10.3390/rs11060617.
|
| [70] |
Williamson CE, Saros JE, Vincent WF et al. Lakes and reservoirs as sentinels,integrators,and regulators of climate change. Limnology and Oceanography,2009,54(6part2):2273-2282. DOI:10.4319/lo.2009.54.6_part_2.2273.
|
| [71] |
Moss B. Cogs in the endless machine: Lakes,climate change and nutrient cycles: A review. Science of the Total Environment,2012,434:130-142. DOI:10.1016/j.scitotenv.2011.07.069.
|
| [72] |
Thackeray SJ, Jones ID, Maberly SC. Long-term change in the phenology of spring phytoplankton: Species-specific responses to nutrient enrichment and climatic change. Journal of Ecology,2008,96(3):523-535. DOI:10.1111/j.1365-2745.2008.01355.x.
|
| [73] |
Winder M, Schindler DE. Climatic effects on the phenology of lake processes. Global Change Biology,2004,10(11):1844-1856. DOI:10.1111/j.1365-2486.2004.00849.x.
|
| [74] |
Conversi A, Peluso T, Fonda-Umani S. Gulf of Trieste: A changing ecosystem. Journal of Geophysical Research: Oceans,2009,114(C3): C03S90. DOI:10.1029/2008JC004763.
|
| [75] |
Chiba S, Tadokoro K, Sugisaki H et al. Effects of decadal climate change on zooplankton over the last 50 years in the western subarctic North Pacific. Global Change Biology,2006,12(5):907-920. DOI:10.1111/j.1365-2486.2006.01136.x.
|
| [76] |
Greve W, Prinage S, Zidowitz H et al. On the phenology of north sea ichthyoplankton. ICES Journal of Marine Science,2005,62(7):1216-1223. DOI:10.1016/j.icesjms.2005.03.011.
|
| [77] |
Palmer SCJ, Odermatt D, Hunter PD et al. Satellite remote sensing of phytoplankton phenology in Lake Balaton using 10 years of MERIS observations. Remote Sensing of Environment,2015,158:441-452. DOI:10.1016/j.rse.2014.11.021.
|
| [78] |
Vantrepotte V, Mélin F. Inter-annual variations in the SeaWiFS global chlorophylla concentration(1997-2007). Deep Sea Research Part I: Oceanographic Research Papers,2011,58(4):429-441. DOI:10.1016/j.dsr.2011.02.003.
|
| [79] |
Winder M, Cloern JE. The annual cycles of phytoplankton biomass. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences,2010,365(1555):3215-3226. DOI:10.1098/rstb.2010.0125.
|
| [80] |
Kostadinov TS, Cabré A, Vedantham H et al. Inter-comparison of phytoplankton functional type phenology metrics derived from ocean color algorithms and earth system models. Remote Sensing of Environment,2017,190:162-177. DOI:10.1016/j.rse.2016.11.014.
|
| [81] |
Henson SA, Robinson I, Allen JT et al. Effect of meteorological conditions on interannual variability in timing and magnitude of the spring bloom in the Irminger Basin, North Atlantic. Deep Sea Research Part I: Oceanographic Research Papers,2006,53(10):1601-1615. DOI:10.1016/j.dsr.2006.07.009.
|
| [82] |
Siegel DA, Doney SC, Yoder JA. The North Atlantic spring phytoplankton bloom and Sverdrup's critical depth hypothesis. Science,2002,296(5568):730-733. DOI:10.1126/science.1069174.
|
| [83] |
Brody SR, Lozier MS, Dunne JP. A comparison of methods to determine phytoplankton bloom initiation. Journal of Geophysical Research: Oceans,2013,118(5):2345-2357. DOI:10.1002/jgrc.20167.
|
| [84] |
Thomalla SJ, Fauchereau N, Swart S et al. Regional scale characteristics of the seasonal cycle of chlorophyll in the Southern Ocean. Biogeosciences,2011,8(10):2849-2866. DOI:10.5194/bg-8-2849-2011.
|
| [85] |
Vargas M, Brown CW, Sapiano MRP. Phenology of marine phytoplankton from satellite ocean color measurements. Geophysical Research Letters,2009,36(1): L01608. DOI:10.1029/2008GL036006.
|
| [86] |
Sapiano MRP, Brown CW, Uz SS et al. Establishing a global climatology of marine phytoplankton phenological characteristics. Journal of Geophysical Research: Oceans,2012,117(C8): C08026. DOI:10.1029/2012JC007958.
|
| [87] |
Rolinski S, Horn H, Petzoldt T et al. Identifying cardinal dates in phytoplankton time series to enable the analysis of long-term trends. Oecologia,2007,153(4):997-1008. DOI:10.1007/s00442-007-0783-2.
|
| [88] |
Mao ZX, Mao ZH, Jamet C et al. Seasonal cycles of phytoplankton expressed by sine equations using the daily climatology from satellite-retrieved chlorophyll-a concentration(1997-2019)over global ocean. Remote Sensing,2020,12(16):2662. DOI:10.3390/rs12162662.
|
| [89] |
Navarro G, Caballero I, Prieto L et al. Seasonal-to-interannual variability of chlorophyll-a bloom timing associated with physical forcing in the Gulf of Cádiz. Advances in Space Research,2012,50(8):1164-1172. DOI:10.1016/j.asr.2011.11.034.
|
| [90] |
Palacz AP, Xue HJ, Armbrecht C et al. Seasonal and inter-annual changes in the surface chlorophyll of the South China Sea. Journal of Geophysical Research: Oceans,2011,116(C9): C09015. DOI:10.1029/2011JC007064.
|
| [91] |
Zhang M, Zhang YL, Qiao FL et al. Shifting trends in bimodal phytoplankton blooms in the North Pacific and North Atlantic Oceans from space with the holo-hilbert spectral analysis. IEEE Journal of Selected Topics in Applied Earth Observations and Remote Sensing,2017,10(1):57-64. DOI:10.1109/JSTARS.2016.2625813.
|
| [92] |
Zhang M, Zhang YL, Shu Q et al. Spatiotemporal evolution of the chlorophyll a trend in the North Atlantic Ocean. Science of the Total Environment,2018,612:1141-1148. DOI:10.1016/j.scitotenv.2017.08.303.
|
| [93] |
Henson SA, Cole HS, Hopkins J et al. Detection of climate change-driven trends in phytoplankton phenology. Global Change Biology,2018,24(1):e101-e111. DOI:10.1111/gcb.13886.
|
| [94] |
Zhang M, Duan HT, Shi XL et al. Contributions of meteorology to the phenology of cyanobacterial blooms: Implications for future climate change. Water Research,2012,46(2):442-452. DOI:10.1016/j.watres.2011.11.013.
|
| [95] |
Wernand MR,van der Woerd HJ, Gieskes WWC. Trends in ocean colour and chlorophyll concentration from 1889 to 2000,worldwide. PLoS One,2013,8(6):e63766. DOI:10.1371/journal.pone.0063766.
|
| [96] |
Thackeray SJ, Henrys PA, Hemming D et al. Phenological sensitivity to climate across taxa and trophic levels. Nature,2016,535:241-245. DOI:10.1038/nature18608.
|
| [97] |
Wang MQ, Hu CM. Satellite remote sensing of pelagic Sargassum macroalgae: The power of high resolution and deep learning. Remote Sensing of Environment,2021,264:112631. DOI:10.1016/j.rse.2021.112631.
|
| [98] |
Lavigne H, D'Ortenzio F, Migon C et al. Enhancing the comprehension of mixed layer depth control on the Mediterranean phytoplankton phenology. Journal of Geophysical Research: Oceans,2013,118(7):3416-3430. DOI:10.1002/jgrc.20251.
|
| [99] |
Siokou-Frangou I, Christaki U, Mazzocchi MG et al. Plankton in the open Mediterranean Sea: A review. Biogeosciences,2010,7(5):1543-1586. DOI:10.5194/bg-7-1543-2010.
|
| [100] |
Tanhua T, Hainbucher D, Schroeder K et al. The Mediterranean Sea system: A review and an introduction to the special issue. Ocean Science,2013,9(5):789-803. DOI:10.5194/os-9-789-2013.
|
| [101] |
Wu J. Wind-stress coefficients over sea surface from breeze to hurricane. Journal of Geophysical Research: Oceans,1982,87(C12):9704-9706. DOI:10.1029/JC087iC12p09704.
|
| [102] |
Yelland M, Taylor PK. Wind stress measurements from the open ocean. Journal of Physical Oceanography,1996,26(4):541-558. DOI:10.1175/1520-0485(1996)0260541:wsmfto>2.0.co;2.
|
| [103] |
Raitsos DE, Lavender SJ, Pradhan Y et al. Coccolithophore bloom size variation in response to the regional environment of the subarctic North Atlantic. Limnology and Oceanography,2006,51(5):2122-2130. DOI:10.4319/lo.2006.51.5.2122.
|
| [104] |
Wasmund N. Occurrence of cyanobacterial blooms in the Baltic Sea in relation to environmental conditions. Internationale Revue Der Gesamten Hydrobiologie Und Hydrographie,1997,82(2):169-184. DOI:10.1002/iroh.19970820205.
|
| [105] |
Legendre L. The significance of microalgal blooms for fisheries and for the export of particulate organic carbon in oceans. Journal of Plankton Research,1990,12(4):681-699. DOI:10.1093/plankt/12.4.681.
|
| [106] |
Basterretxea G, Font-Muñoz JS, Salgado-Hernanz PM et al. Patterns of chlorophyll interannual variability in Mediterranean biogeographical regions. Remote Sensing of Environment,2018,215:7-17. DOI:10.1016/j.rse.2018.05.027.
|
| [107] |
Katara I, Illian J, Pierce GJ et al. Atmospheric forcing on chlorophyll concentration in the Mediterranean. Hydrobiologia,2008,612(1):33-48. DOI:10.1007/s10750-008-9492-z.
|
| [108] |
Irminger Basin NA. Effect of meteorological conditions on interannual variability in timing and magnitude of the spring bloom in the. Deep-Sea Research,2006,1(53):1601-1615.
|
| [109] |
Colella S, Falcini F, Rinaldi E et al. Mediterranean ocean colour chlorophyll trends. PLoS One,2016,11(6):e0155756. DOI:10.1371/journal.pone.0155756.
|
| [110] |
Venrick EL. Phytoplankton seasonality in the central North Pacific: The endless summer reconsidered. Limnology and Oceanography,1993,38(6):1135-1149. DOI:10.4319/lo.1993.38.6.1135.
|
| [111] |
Gregg WW, Ginoux P, Schopf PS et al. Phytoplankton and iron: Validation of a global three-dimensional ocean biogeochemical model. Deep Sea Research Part II: Topical Studies in Oceanography,2003,50(22/23/24/25/26):3143-3169. DOI:10.1016/j.dsr2.2003.07.013.
|
| [112] |
Moore JK, Doney SC. Iron availability limits the ocean nitrogen inventory stabilizing feedbacks between marine denitrification and nitrogen fixation. Global Biogeochemical Cycles,2007,21(2): GB2001. DOI:10.1029/2006GB002762.
|
| [113] |
Song KS, Fang C, Jacinthe PA et al. Climatic versus anthropogenic controls of decadal trends(1983-2017)in algal blooms in lakes and reservoirs across China. Environmental Science & Technology,2021,55(5):2929-2938. DOI:10.1021/acs.est.0c06480.
|
| [114] |
Kakouei K, Kraemer BM, Anneville O et al. Phytoplankton and cyanobacteria abundances in mid-21st century lakes depend strongly on future land use and climate projections. Global Change Biology,2021,27(24):6409-6422. DOI:10.1111/gcb.15866.
|
| [115] |
LeBlanc RT, Brown RD, FitzGibbon JE. Modeling the effects of land use change on the water temperature in unregulated urban streams. Journal of Environmental Management,1997,49(4):445-469. DOI:10.1006/jema.1996.0106.
|
| [116] |
Tang LF, Yang K, Shang CX et al. Spatial impact of urban expansion on lake surface water temperature based on the perspective of watershed scale. Frontiers in Environmental Science,2022,10:991502. DOI:10.3389/fenvs.2022.991502.
|
| [117] |
Liu YH, Xu YM, Zhang FM et al. A preliminary study on the influence of Beijing urban spatial morphology on near-surface wind speed. Urban Climate,2020,34:100703. DOI:10.1016/j.uclim.2020.100703.
|
| [118] |
Wynne TT, Stumpf RP, Litaker RW et al. Cyanobacterial bloom phenology in Saginaw Bay from MODIS and a comparative look with western Lake Erie. Harmful Algae,2021,103:101999. DOI:10.1016/j.hal.2021.101999.
|
| [119] |
Yang SB, Chen XL, Lu JZ et al. Impacts of agricultural topdressing practices on cyanobacterial bloom phenology in an early eutrophic Plateau Lake, China. Journal of Hydrology,2021,594:125952. DOI:10.1016/j.jhydrol.2020.125952.
|
| [120] |
Kononen K. Dynamics of the toxic cyanobacterial blooms in the Baltic Sea. Mar Res,1992,261:3-36.
|
| [121] |
Kiirikki M, Inkala A, Kuosa H et al. Evaluating the effects of nutrient load reductions on the biomass of toxic nitrogen-fixing cyanobacteria in the Gulf of Finland, Baltic Sea. Boreal Environment Research,2001,6(2):131-146.
|
| [122] |
Lilover MJ, Stips A. The variability of parameters controlling the cyanobacteria bloom biomass in the Baltic Sea. Journal of Marine Systems,2008,74: S108-S115. DOI:10.1016/j.jmarsys.2008.03.029.
|
| [123] |
Barlow RG, Mantoura RFC, Gough MA et al. Pigment signatures of the phytoplankton composition in the northeastern Atlantic during the 1990 spring bloom. Deep Sea Research Part II: Topical Studies in Oceanography,1993,40(1/2):459-477. DOI:10.1016/0967-0645(93)90027-k.
|
| [124] |
Moore JK, Doney SC, Lindsay K. Upper ocean ecosystem dynamics and iron cycling in a global three-dimensional model. Global Biogeochemical Cycles,2004,18(4): GB4028. DOI:10.1029/2004gb002220.
|
| [125] |
Jia WX, Zhao SQ, Zhang XY et al. Urbanization imprint on land surface phenology: The urban-rural gradient analysis for Chinese cities. Global Change Biology,2021,27(12):2895-2904. DOI:10.1111/gcb.15602.
|
